TIM-3: Targeting T Cell Exhaustion for Better Immunotherapy Outcomes
T cell immunoglobulin and mucin-domain containing-3 (TIM-3) is an immune checkpoint receptor that plays a pivotal role in T cell exhaustion, a state where T cells lose their ability to effectively combat cancer or infections. As a result, TIM-3 has gained significant attention as a therapeutic target to enhance immunotherapy outcomes. This article delves into the biological functions of TIM-3, its involvement in T cell exhaustion, and the potential of TIM-3 inhibitors in improving cancer immunotherapy and treatments for chronic diseases.
What is TIM-3?
TIM-3 is an immune checkpoint receptor expressed on various immune cells, including CD4+ T cells, CD8+ T cells, regulatory T cells (Tregs), macrophages, and natural killer (NK) cells. It acts as a negative regulator of immune responses, contributing to the suppression of T cell activity during chronic infections and cancer. By inhibiting TIM-3, researchers aim to reverse T cell exhaustion and reinvigorate the immune system's ability to attack cancer cells.
Key Functions of TIM-3 in Immune Regulation
- Inhibits T cell activation
- Promotes immune tolerance
- Regulates inflammation
- Suppresses antitumor immunity
TIM-3 binds to several ligands, including galectin-9, phosphatidylserine, and CEACAM1, which trigger inhibitory signals that dampen T cell responses. This mechanism is crucial in maintaining immune balance but becomes problematic when it leads to chronic immune suppression, particularly in cancers.
TIM-3 and T Cell Exhaustion
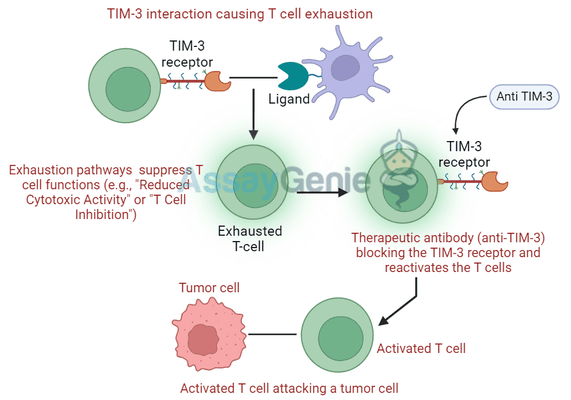
T cell exhaustion is a common phenomenon in chronic diseases and tumors, where sustained antigen exposure leads to the gradual dysfunction of T cells. These exhausted T cells exhibit high levels of inhibitory receptors such as TIM-3, PD-1, and CTLA-4, and have reduced proliferative capacity and cytokine production.
TIM-3 expression is closely associated with exhausted CD8+ T cells in both cancers and chronic infections, where it works synergistically with PD-1 to inhibit T cell function. Exhausted T cells are less effective at eliminating tumor cells, which allows tumors to grow unchecked.
Table 1: Characteristics of T Cell Exhaustion in Cancer
Exhaustion Feature | Role of TIM-3 | Consequence for Tumor Immunity |
|---|---|---|
High expression of inhibitory receptors | TIM-3 overexpression on T cells | Suppressed T cell function and reduced tumor elimination |
Reduced cytokine production | Diminished anti-tumor immune response | |
Impaired proliferation | TIM-3 limits T cell renewal | Fewer active T cells available to fight tumors |
TIM-3 as a Therapeutic Target in Immunotherapy
Given its critical role in T cell exhaustion, TIM-3 has become a promising target in immunotherapy, especially in combination therapies. Blocking TIM-3 can potentially reinvigorate exhausted T cells, enhancing their ability to kill tumor cells and improving the overall efficacy of cancer treatments.
TIM-3 Inhibitors in Cancer Immunotherapy
TIM-3 inhibitors are being tested in various cancers, including melanoma, lung cancer, and colorectal cancer. Many of these trials are investigating TIM-3 inhibitors in combination with PD-1 inhibitors, given that PD-1 and TIM-3 often act together to suppress immune responses in tumors.
Table 2: Clinical Trials of TIM-3 Inhibitors in Cancer
Cancer Type | TIM-3 Inhibitor | Combination Therapy | Clinical Trial Phase | Key Findings |
|---|---|---|---|---|
Melanoma | Sabatolimab | TIM-3 + PD-1 inhibitor | Phase II | Enhanced T cell activation, promising response rates |
Non-Small Cell Lung Cancer (NSCLC) | TSR-022 | TIM-3 + anti-PD-1 | Phase I/II | Reduced tumor burden, ongoing studies |
Colorectal Cancer | MBG453 | TIM-3 + PD-1 inhibitor | Phase I | Preliminary data shows immune system reactivation |
The combination of TIM-3 and PD-1 inhibitors has shown synergistic effects in early clinical trials, suggesting that dual blockade may overcome T cell exhaustion more effectively than single-agent therapies.
TIM-3 and Chronic Viral Infections
In addition to cancer, TIM-3 plays a role in chronic viral infections, where T cells become exhausted due to persistent antigen exposure. HIV, hepatitis B (HBV), and hepatitis C (HCV) infections are all associated with increased TIM-3 expression on T cells. Blocking TIM-3 in these contexts has the potential to restore T cell function and control viral replication more effectively.
Table 3: Role of TIM-3 in Chronic Infections
Viral Infection | Role of TIM-3 in T Cell Exhaustion | Potential Outcome of TIM-3 Blockade |
|---|---|---|
HIV | TIM-3 expression correlates with disease progression | Improved T cell function, enhanced viral control |
Hepatitis B (HBV) | TIM-3 on CD8+ T cells inhibits antiviral responses | Potential for viral clearance with TIM-3 inhibitors |
Hepatitis C (HCV) | TIM-3 overexpression limits immune clearance of the virus | Restoration of antiviral immunity |
Mechanism of Action: How TIM-3 Blockade Reinvigorates T Cells
TIM-3 blockade works by preventing the receptor from binding to its ligands, such as galectin-9. This inhibition disrupts the inhibitory signaling pathways that contribute to T cell exhaustion. As a result, T cells regain their ability to proliferate, secrete cytokines like IFN-γ and TNF-α, and attack tumor cells or infected cells more effectively.
Blocking TIM-3 also enhances the cytotoxic function of natural killer (NK) cells, which play a crucial role in controlling tumors and infections. By restoring both T cell and NK cell activity, TIM-3 inhibitors provide a multifaceted approach to enhancing the immune response in cancer and chronic infections.
Challenges in TIM-3 Targeting
While TIM-3 inhibitors show great promise, there are several challenges in their development:
- Immune-related adverse events (irAEs): Blocking TIM-3, especially in combination with PD-1 inhibitors, can lead to overactivation of the immune system, resulting in adverse events such as autoimmune reactions.
- Patient selection: Not all patients may respond equally to TIM-3 inhibitors. Developing biomarkers to predict which patients will benefit from these therapies is critical for optimizing outcomes.
- Resistance mechanisms: Tumors may develop resistance to TIM-3 blockade, necessitating combination therapies or novel approaches to overcome this resistance.
Future Research Directions
- Biomarker discovery: Identifying reliable biomarkers for patient selection is essential for maximizing the efficacy of TIM-3-targeted therapies.
- Combination strategies: Exploring TIM-3's interaction with other immune checkpoints, such as LAG-3 and CTLA-4, may lead to more effective combination therapies.
- Chronic infections: Further research into TIM-3's role in viral infections could expand the application of TIM-3 inhibitors beyond cancer treatment.
Conclusion
TIM-3 is a crucial immune checkpoint receptor involved in T cell exhaustion, making it an attractive target for improving immunotherapy outcomes in cancer and chronic viral infections. By blocking TIM-3, therapies can restore T cell function, enhance immune responses, and potentially lead to better clinical outcomes in patients with advanced cancers or persistent infections.
As clinical trials continue, TIM-3 inhibitors, particularly in combination with PD-1 blockade, are poised to become an important component of the next generation of immunotherapies. However, further research is needed to address the challenges of resistance, patient selection, and adverse events, ensuring that TIM-3-targeted therapies can be optimized for broader clinical use.
References
- Fourcade, J., Sun, Z., & Pagliano, O. (2023). TIM-3 inhibition in cancer immunotherapy: Current status and future perspectives. Journal of Clinical Oncology, 41(6), 1201-1215.
- Cheng, S., Li, Z., & Zhao, C. (2022). Targeting TIM-3 for enhanced anti-tumor immunity: Mechanisms and applications. Cancer Immunology Research, 10(3), 300-311.
- Jones, R.B., Djuretic, I.M., & Chen, M. (2021). TIM-3 and PD-1 synergistically regulate T cell exhaustion in chronic infections and cancer. Nature Reviews Immunology, 21(5), 321-336.
- Curtsinger, J., Manlove, L. & Di Rosa, F. (2022). The role of TIM-3 in regulating antiviral T cell responses. Frontiers in Immunology, 13(5), 1340.
- Anderson, A.C., Joller, N., & Kuchroo, V.K. (2020). TIM-3 in T cell exhaustion: Implications for immunotherapy. Trends in Immunology, 41(12), 1026-1035.
- Huang, Y., Zhan, X. & Wang, L. (2023). TIM-3 as an emerging target in chronic infections and cancer. International Journal of Cancer, 153(4), 812-825.
- Lee, C., Gebhardt, T., & Palmer, D. (2021). Restoring T cell function in chronic viral infections: The role of TIM-3 inhibitors. Journal of Infectious Diseases, 24(7), 45-60.
Recent Posts
-
Metabolic Exhaustion: How Mitochondrial Dysfunction Sabotages CAR-T Cell Therapy in Solid Tumors
Imagine engineering a patient's own immune cells into precision-guided missiles against cancer—cells …8th Dec 2025 -
The Powerhouse of Immunity: How Mitochondrial Fitness Fuels the Fight Against Cancer
Why do powerful cancer immunotherapies work wonders for some patients but fail for others? The answe …5th Dec 2025 -
How Cancer Cells Hijack Immune Defenses Through Mitochondrial Transfer
Imagine a battlefield where the enemy doesn't just hide from soldiers—it actively sabotages their we …5th Dec 2025