CD25 and Tregs: Understanding the Role of Regulatory T Cells in Tumor Immunity
Regulatory T cells (Tregs) play a crucial role in maintaining immune balance by suppressing excessive immune responses that could lead to tissue damage. However, in the context of cancer, Tregs can be problematic because they also suppress the immune system’s ability to attack and eliminate tumors. One of the key markers of Tregs is CD25, which is the alpha chain of the IL-2 receptor. This article explores the role of CD25 and Tregs in tumor immunity, shedding light on how these cells contribute to tumor growth and how they can be targeted to improve cancer immunotherapy outcomes.
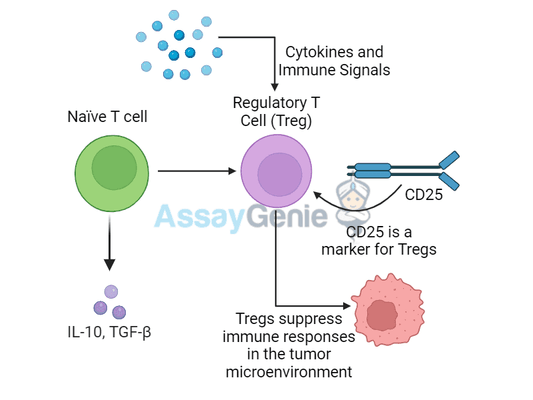
What Are Regulatory T Cells (Tregs)?
Tregs are a specialized subset of CD4+ T cells that function to suppress immune responses and maintain immune homeostasis. Their primary role is to prevent autoimmune reactions and chronic inflammation by regulating other immune cells, particularly effector T cells that might otherwise attack healthy tissues.
Key Functions of Tregs in Immune Regulation
- Immune suppression:
Tregs inhibit the activation and proliferation of other immune cells, suchas effector T cells, NK cells, and dendritic cells. - Cytokine production:
Tregs secrete anti-inflammatory cytokines like IL-10 and TGF-β, which help dampen immune responses. - Metabolic disruption:
Tregs can consume large amounts of IL-2, a cytokine crucial for T cell activation, thereby limiting effector T cell proliferation.
Table 1: Key Features of Tregs in Immune Regulation
Feature | Function |
|---|---|
Suppression of effector T cells | Inhibits T cell activation and cytokine production |
Secretion of IL-10 and TGF-β | Produces anti-inflammatory cytokines to limit immune responses |
Expression of CD25 | High-affinity IL-2 receptor, which consumes IL-2 and limits effector T cell proliferation |
CD25: The Alpha Chain of the IL-2 Receptor
Functions of CD25 in Tregs:
- IL-2 consumption:
CD25 allows Tregs to outcompete other immune cells for IL-2, thus depriving effector T cells of this key growth factor. - Treg survival:
The binding of IL-2 to CD25 is critical for Treg survival and function, ensuring that these cells can effectively suppress immune responses. - Tumor immune evasion:
In the tumor microenvironment, high CD25 expression on Tregs helps tumors evade the immune system by suppressing anti-tumor immune responses.
Table 2: CD25 in Tregs and Effector T Cells
Cell Type | CD25 Expression | Function |
|---|---|---|
Regulatory T cells | High | Facilitates IL-2 consumption and Treg survival |
Effector T cells | Low to moderate (activation-dependent) | Depends on IL-2 for proliferation, often outcompeted by Tregs |
Tregs in the Tumor Microenvironment
In the context of cancer, Tregs are often recruited into the tumor microenvironment (TME), where they suppress immune responses that would otherwise target and destroy tumor cells. The presence of high numbers of Tregs in tumors is associated with poor prognosis in many types of cancer because these cells prevent the immune system from mounting an effective anti-tumor response.
Mechanisms by Which Tregs Promote Tumor Growth:
By inhibiting effector T cells, Tregs reduce the immune system’s ability to recognize and destroy cancer cells.
Tregs consume IL-2, depriving effector T cells of a key growth factor and further limiting anti-tumor immunity.
Table 3: Role of Tregs in Tumor Immune Evasion
Mechanism | Impact on Tumor Immunity |
|---|---|
Suppression of effector T cells | Inhibits anti-tumor immune responses, allowing tumor cells to grow |
Production of IL-10 and TGF-β | Dampens inflammation and cytotoxic activity in the TME |
High consumption of IL-2 | Reduces effector T cell proliferation and immune surveillance |
Targeting CD25 and Tregs in Cancer Immunotherapy
1. CD25-Targeting Antibodies
Monoclonal antibodies that target CD25 can selectively deplete Tregs in the tumor microenvironment, reducing their suppressive effects and enhancing the activity of effector T cells. However, these therapies must be designed carefully to avoid depleting activated effector T cells that also express CD25 during immune responses.
2. Low-dose IL-2 Therapy
Low-dose IL-2 has been explored as a therapy to preferentially expand Tregs in autoimmune diseases, but high-dose IL-2 could promote effector T cell activity. Therefore, balancing IL-2 therapy is critical depending on the disease context.
3. Checkpoint Inhibitors and Tregs
Checkpoint inhibitors such as anti-PD-1 and anti-CTLA-4 work by blocking inhibitory signals on T cells, potentially overcoming Treg-mediated suppression. When combined with therapies targeting CD25 or Tregs, these inhibitors may provide a synergistic effect, unleashing a more robust anti-tumor immune response.
Table 4: Therapeutic Approaches Targeting Tregs in Cancer
Therapy Type | Mechanism | Status |
|---|---|---|
CD25-targeting antibodies | Deplete Tregs to enhance anti-tumor immune responses | Ongoing clinical trials |
Low-dose IL-2 therapy | Balances Treg expansion or effector T cell activation | Early-stage research |
Checkpoint inhibitors + Treg depletion | Combines Treg depletion with immune checkpoint blockade | Approved in several cancers |
Challenges and Future Directions
Targeting Tregs and CD25 in cancer therapy is a promising approach, but several challenges remain:
- Selectivity:
Therapies targeting CD25 must be carefully designed to selectively depleteTregs without affecting effector T cells, which also express CD25 upon activation. - Tumor-specific Treg depletion: Systemic depletion of Tregs could lead to unwanted autoimmunity. Tumor-specific Treg targeting is needed to minimize off-target effects.
- Resistance mechanisms:
Tumors may develop compensatory mechanisms to suppress immune responses, even when Tregs are depleted. Combination therapies may be necessary to overcome such resistance.
Future Research Directions
Combination strategies: Investigating the use of CD25-targeting antibodies in combination with checkpoint inhibitors or CAR-T cell therapies to boost anti-tumor immunity.
Tumor microenvironment modulation: Exploring ways to modulate the TME to make it less hospitable for Tregs while enhancing effector T cell function.
Conclusion
Tregs, marked by high CD25 expression, are essential for maintaining immune balance, but their role in tumor immune evasion makes them a critical target in cancer therapy. By suppressing the immune system’s ability to attack cancer cells, Tregs enable tumor growth and progression. Understanding the role of CD25 and Tregs in the tumor microenvironment has opened new avenues for immunotherapeutic approaches aimed at depleting or inhibiting Tregs to enhance anti-tumor immunity. With further research and refinement, therapies targeting Tregs and CD25 may significantly improve outcomes for cancer patients.
References
- Campbell, D.J., & Koch, M.A. (2022). Targeting regulatory T cells in cancer: Emerging therapies and challenges. Nature Immunology, 23(6), 709-718.
- Sakaguchi, S., Mikami, N., & Wing, J.B. (2021). Regulatory T cells and human disease. Annual Review of Immunology, 39, 453-481.
- Tang, Q., & Bluestone, J.A. (2020). Regulatory T-cell therapy in transplantation: Moving to the clinic. Nature Reviews Immunology, 20(2), 143-155.
- Sharma, P., & Allison, J.P. (2020). The future of immune checkpoint therapy. Science, 348(6230), 56-61.
- Kim, H.J., & Cantor, H. (2022). CD25-targeted therapies: A new strategy for cancer immunotherapy. Cancer Research, 82(4), 765-775.
- Wolf, D., & Anderson, A.C. (2020). Tregs and CD25: Targeting regulatory T cells in cancer. Journal of Clinical Oncology, 38(13), 1553-1561.
Recent Posts
-
Metabolic Exhaustion: How Mitochondrial Dysfunction Sabotages CAR-T Cell Therapy in Solid Tumors
Imagine engineering a patient's own immune cells into precision-guided missiles against cancer—cells …8th Dec 2025 -
The Powerhouse of Immunity: How Mitochondrial Fitness Fuels the Fight Against Cancer
Why do powerful cancer immunotherapies work wonders for some patients but fail for others? The answe …5th Dec 2025 -
How Cancer Cells Hijack Immune Defenses Through Mitochondrial Transfer
Imagine a battlefield where the enemy doesn't just hide from soldiers—it actively sabotages their we …5th Dec 2025