Cobolimab: Unveiling TIM-3’s Role in Cancer Immunotherapy
Quick Facts About Cobolimab
What is Cobolimab?
Cobolimab is a monoclonal antibody that targets TIM-3, a checkpoint protein involved in immune regulation, especially in cancer and autoimmune diseases.
How does Cobolimab work?
By inhibiting TIM-3, Cobolimab reactivates T-cells, boosting the immune system’s ability to identify and destroy cancer cells.
What is Cobolimab’s significance in GSK’s research?
As part of GSK’s immuno-oncology pipeline, Cobolimab is being investigated for its potential in combination therapies to improve cancer treatment outcomes.
1.) Understanding Cobolimab
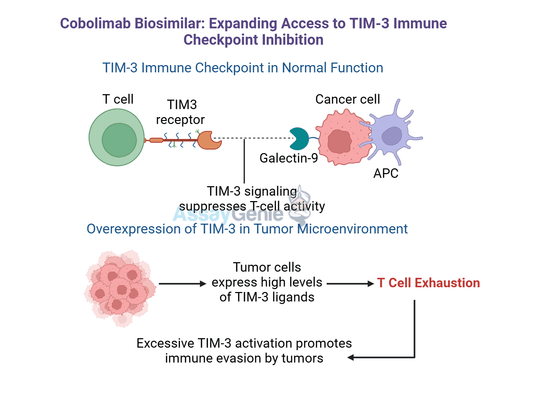
Cobolimab is a novel monoclonal antibody developed to enhance cancer immunotherapy by targeting T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), a key immune checkpoint receptor involved in regulating immune responses. TIM-3 is expressed on various immune cells, including T cells, dendritic cells, and macrophages, where it plays a crucial role in maintaining immune balance by dampening immune activity under normal conditions. However, many tumors exploit this pathway to suppress immune responses, enabling immune evasion and unchecked growth.
Cobolimab blocks TIM-3, thereby restoring the immune system's capacity to recognize and attack tumor cells. This inhibition reinvigorates T-cell activity, enhances dendritic cell function, and promotes a more robust anti-tumor response. Cobolimab’s mechanism of action is particularly effective when combined with other immune checkpoint inhibitors, such as anti-PD-1 or anti-CTLA-4 therapies, which target complementary pathways to further release immune suppression. This combination approach provides a synergistic effect, boosting overall immune-mediated tumor destruction.
Preclinical and clinical studies suggest that Cobolimab has significant potential in the treatment of various solid tumors and hematological malignancies. By overcoming tumor-induced immune suppression, Cobolimab represents a promising strategy to advance cancer immunotherapy, offering new hope for patients with hard-to-treat cancers.
Prefer to Listen? Check out the Cobolimab Podcast Episode
2.) Mechanism of Action of Cobolimab
Cobolimab functions by targeting and inhibiting T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), a key immune checkpoint receptor involved in immune regulation. TIM-3 interacts with various ligands, such as Galectin-9, phosphatidylserine, and HMGB1, which collectively contribute to the suppression of T-cell activation and immune response. These interactions are often exploited by tumors to evade immune surveillance, creating an immunosuppressive microenvironment that promotes tumor growth and progression.
By blocking TIM-3’s interaction with its ligands, Cobolimab prevents the suppression of T cells and restores their ability to sustain anti-tumor activity. This mechanism not only reactivates exhausted T cells, which are critical for targeting tumor cells, but also improves the function of other immune cells, such as dendritic cells, which play a key role in antigen presentation and immune priming. This broad immune activation contributes to a more effective anti-tumor response.
Cobolimab’s mechanism complements other immune checkpoint inhibitors, such as PD-1/PD-L1 therapies, which address different pathways of immune suppression. The combination of TIM-3 inhibition with PD-1 blockade enhances immune system reactivation through synergistic effects, resulting in improved anti-tumor responses. Preclinical and early clinical studies have demonstrated this potential, showing promising results in reducing tumor burden and prolonging survival in several cancer models.
This synergy positions Cobolimab as a valuable addition to combination immunotherapy strategies, offering a comprehensive approach to overcoming immune suppression and improving outcomes for patients with difficult-to-treat cancers.

3.) Clinical Applications of Cobolimab
Cobolimab is being actively studied for its potential to treat a range of cancers, including solid tumors such as melanoma, non-small cell lung cancer (NSCLC), and colorectal cancer, as well as hematologic malignancies like lymphoma. Its targeted inhibition of TIM-3, an immune checkpoint receptor implicated in tumor-driven immune suppression, makes Cobolimab a promising candidate for enhancing the effectiveness of cancer immunotherapy.
Current research focuses on Cobolimab’s role in combination therapy, particularly with PD-1/PD-L1 inhibitors, which have revolutionized cancer treatment but face limitations due to resistance in some patients. Tumors often exploit multiple immune checkpoint pathways, including TIM-3, to evade immune detection. By combining Cobolimab with PD-1/PD-L1 therapies, researchers aim to overcome this resistance by simultaneously targeting complementary immune-suppressive mechanisms, leading to a more robust anti-tumor response.
Preclinical and early clinical trials have shown encouraging results, with Cobolimab demonstrating the potential to restore T-cell activity, enhance immune system reactivation, and reduce tumor progression when used in combination therapies. Its ability to synergize with other immunotherapies offers a promising strategy to treat cancers that remain refractory to existing approaches, potentially expanding the benefits of immunotherapy to a broader patient population.
While clinical data are still evolving, Cobolimab’s innovative approach provides hope for addressing unmet needs in oncology and improving outcomes for patients with challenging cancers.
4.) Advancing Research on Cobolimab with Biosimilars
What is a Biosimilar?
A biosimilar is a highly similar alternative to an original biologic drug, designed to match its safety, purity, and efficacy. Biosimilars provide researchers with accessible tools to study biological pathways and drug effects without relying on proprietary drugs.
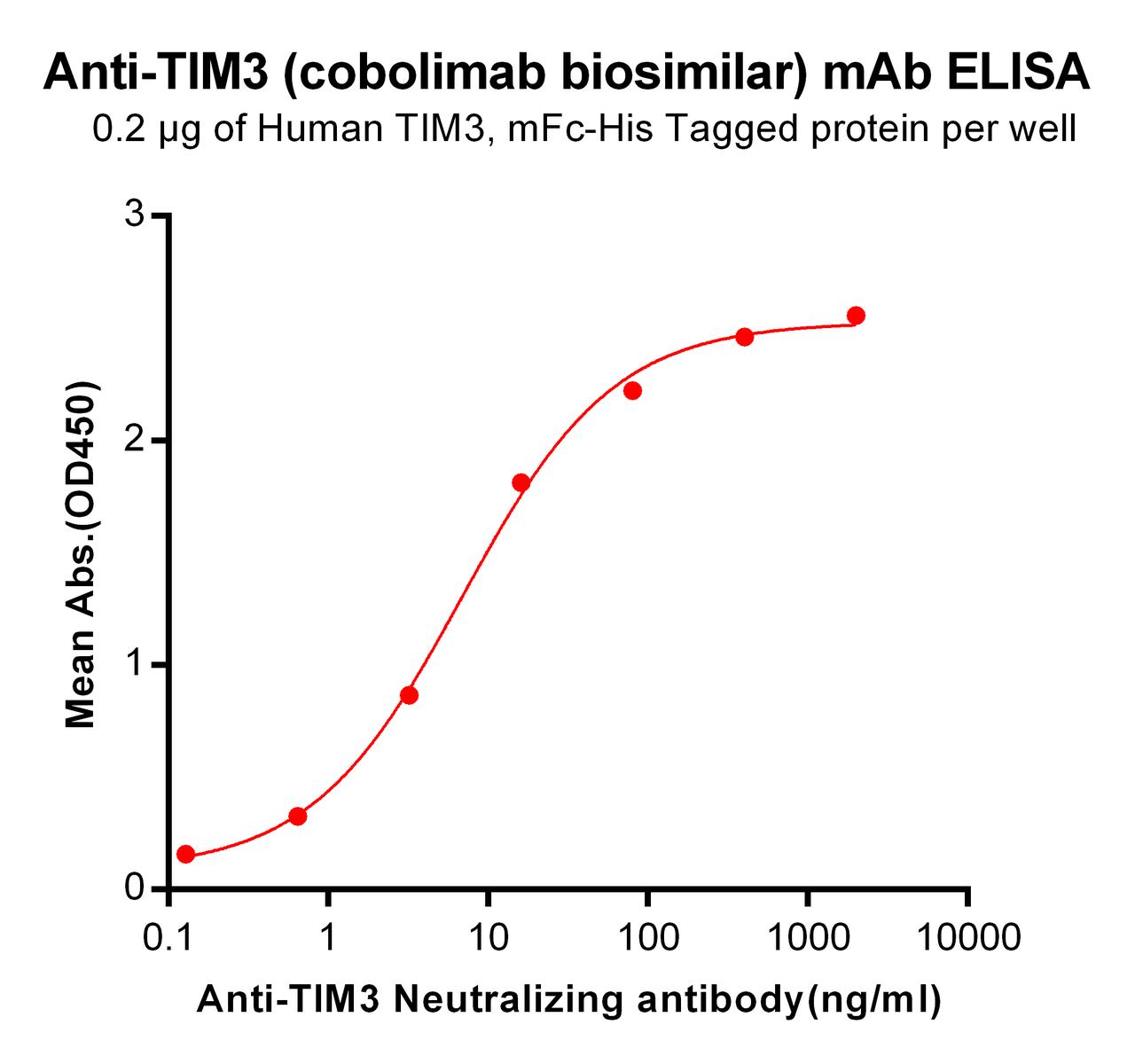
| Cobolimab (Anti-TIM3) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | TIM3 |
| Reactivity: | Human |
Cobolimab Biosimilar: A Tool for Innovation
Our Cobolimab biosimilar is an essential resource for researchers studying TIM-3. Designed for research use only, it enables precise exploration of Cobolimab’s mechanisms and applications in various experimental settings.
Key Benefits of the Cobolimab Biosimilar
- Cost-Effective Access: Provides a more affordable option for studying TIM-3-related pathways.
- High Reliability: Accurately mimics the original drug’s specificity and binding properties, ensuring dependable results.
- Wide Applicability: Supports diverse research needs, from in vitro experiments to preclinical studies.
Note: This biosimilar is strictly for research purposes and is not intended for clinical or therapeutic use.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Miren Ruiz de Eguilaz, PhD
Miren Ruiz de Eguilaz, PhD, has an extensive academic background, earning a BSc in Biology from UPV/EHU, an MSc in Biotechnology from the University of Oviedo, and a PhD in Chemistry from Dublin City University (DCU). Miren’s expertise lies in biosensor technology and bacterial diagnostics. She currently serves as a Product Manager at Assay Genie.
Recent Posts
-
What Are Oligodendrocytes? Functions, Markers & Disease Links
What Are Oligodendrocytes? Functions, Markers & Disease LinksOligodendrocytes are pivo …24th Sep 2025 -
Complete T Helper Cell Guide: Th1, Th2, Th17 & Functions - Your Ultimate Resource from Assay Genie
Complete T Helper Cell Guide: Th1, Th2, Th17 & Functions - Your Ultimate Resource from …27th Aug 2025 -
Apoptosis Unveiled: Your Complete Guide to Intrinsic & Extrinsic Pathways
Apoptosis Unveiled: Your Complete Guide to Intrinsic & Extrinsic PathwaysAt Assay Geni …27th Aug 2025