Tigatuzumab Biosimilar: Harnessing DR5 for Targeted Cancer Therapy
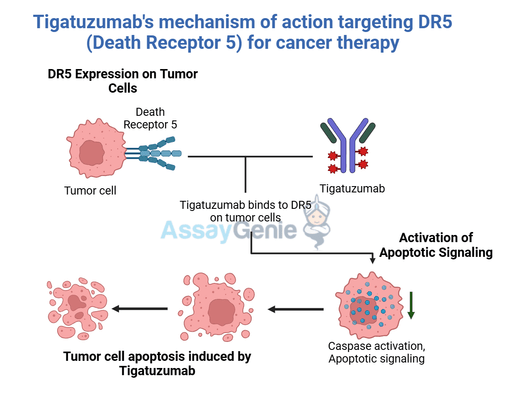
Tigatuzumab is a monoclonal antibody targeting death receptor 5 (DR5), a member of the tumor necrosis factor receptor (TNFR) superfamily. By activating DR5, Tigatuzumab induces apoptosis selectively in cancer cells, making it a promising treatment for solid tumors such as breast cancer, lung cancer, and pancreatic cancer. The biosimilar offers an affordable alternative, broadening access to DR5-targeted therapies.
This article explores the mechanism of action, clinical applications, and benefits of Tigatuzumab biosimilar in oncology.
1. Understanding DR5 and Its Role in Cancer
What is DR5?
Death receptor 5 (DR5) is a pro-apoptotic receptor expressed on the surface of cancer cells. It is:
- Upregulated in Tumors: Found at higher levels in many cancer types, including breast, lung,
and colorectal cancers. - Involved in Apoptosis: Activated by tumor necrosis factor-related apoptosis-inducing ligand
(TRAIL) to trigger programmed cell death.
Why Target DR5?
- Tumor-Selective Action: DR5 activation induces apoptosis primarily in cancer cells, sparing normal tissues.
- Overcomes Resistance: Restores apoptotic pathways often deregulated in cancer cells.
2. Tigatuzumab Biosimilar: A Cost-Effective Solution
Features of the Biosimilar
The Tigatuzumab biosimilar provides the same efficacy and safety as the original antibody but at a reduced cost, enhancing its accessibility.
- Target: DR5 on cancer cells.
- Mechanism: Promotes apoptosis in tumor cells while minimizing toxicity to normal tissues.
- Affordability: Expands access to advanced cancer treatments, particularly in resource-limited settings.
3. Mechanism of Action
Step | Details |
|---|---|
DR5 Binding | The biosimilar binds specifically to DR5 on the surface of cancer cells. |
Apoptosis Activation | Activates DR5 signaling pathways, leading to caspase activation and programmed cell death. |
Immune Activation | Enhances immune-mediated tumor clearance through antibody-dependent cellular cytotoxicity (ADCC). |
Tumor Selectivity | Exploits DR5 overexpression in cancer cells, minimizing off-target effects. |
4. Clinical Applications
Solid Tumors
Triple-Negative Breast Cancer (TNBC)
- Effective in DR5-overexpressing TNBC, a challenging cancer subtype with limited therapeutic options.
Non-Small Cell Lung Cancer (NSCLC)
- Promotes apoptosis in lung cancer cells, particularly when combined with standard chemotherapy.
Pancreatic Cancer
- Demonstrates potential in targeting chemoresistant pancreatic adenocarcinoma by inducing apoptosis in DR5-positive cells.
Combination Therapy Potential
- Tigatuzumab biosimilar is synergistic with chemotherapeutic agents and immune checkpoint inhibitors, enhancing therapeutic efficacy.
5. Benefits of Tigatuzumab Biosimilar
Targeted Apoptosis
Selectively induces apoptosis in DR5-overexpressing cancer cells, sparing healthy tissues.
Cost-Effective Access
Provides a more affordable option for DR5-targeted therapy, improving global accessibility.
Broad Applications
Demonstrates efficacy across a range of solid tumors, including breast, lung, and pancreatic cancers.
6. Challenges and Considerations
Resistance Mechanisms
- Tumors may develop resistance by downregulating DR5 or altering downstream apoptotic pathways.
- Combination therapies can mitigate resistance risks.
Adverse Effects
- Infusion Reactions: Common but manageable with corticosteroids and antihistamines.
- Hepatotoxicity: Requires liver function monitoring in patients with pre-existing conditions.
7. Comparison: Tigatuzumab vs. Biosimilar
Feature | Enavatuzumab | Biosimilar |
|---|---|---|
Target | DR5 on cancer cells. | DR5 on cancer cells. |
Mechanism | Activates DR5-mediated apoptosis and facilitates ADCC. | Activates DR5-mediated apoptosis and facilitates ADCC. |
Indications | Breast, lung, pancreatic, and other DR5-positive cancers. | Breast, lung, pancreatic, and other DR5-positive cancers. |
Efficacy | Proven in clinical trials. | Equivalent in preclinical and clinical studies. |
Cost | High | Reduced, improving accessibility. |
8. Future Directions
Expanded Indications
- Investigating efficacy in other DR5-overexpressing cancers, such as colorectal and ovarian cancers.
Combination Therapies
- Checkpoint Inhibitors: Enhancing immune-mediated tumor clearance with PD-1/PD-L1 inhibitors.
- Radiotherapy: Combining Tigatuzumab with radiation to increase tumor immunogenicity.
9. Summary Table
Aspect | Details |
|---|---|
Target | DR5, a pro-apoptotic receptor overexpressed in cancer cells. |
Primary Use | Treating DR5-positive solid tumors such as breast, lung, and pancreatic cancers. |
Mechanism of Action | Activates apoptosis and enhances immune-mediated tumor clearance. |
Biosimilar Benefits | Affordable, accessible, and clinically equivalent to Tigatuzumab. |
Conclusion
The Tigatuzumab biosimilar offers a targeted approach to treating DR5-positive cancers, selectively inducing apoptosis in tumor cells while minimizing off-target effects. Its cost-effective nature ensures broader accessibility, making it a valuable tool in the fight against aggressive cancers.
References
- Soria, J.C., et al., 2011. DR5-targeting monoclonal antibody Tigatuzumab in cancer therapy. Cancer Cell, 20(2), pp.147-159.
- ClinicalTrials.gov, 2023. Studies on Tigatuzumab and biosimilar therapies. Available at www.clinicaltrials.gov.
- European Medicines Agency (EMA), 2023. Guidelines for biosimilar development in oncology. Available at www.ema.europa.eu.
- Ashkenazi, A., et al., 2008. Targeting death receptors in cancer: Mechanistic insights and clinical potential. Nature Reviews Cancer, 8(6), pp.417-425.
- Herbst, R.S., et al., 2020. Advances in DR5-targeted therapies: The role of Tigatuzumab. The Oncologist, 25(5), pp.390-398.
Recent Posts
-
Tigatuzumab Biosimilar: Harnessing DR5 for Targeted Cancer Therapy
Tigatuzumab is a monoclonal antibody targeting death receptor 5 (DR5), a member of the …17th Dec 2025 -
Siltuximab: Exploring IL-6 Inhibition in Castleman’s Disease and Research
Quick Facts About SiltuximabWhat is Siltuximab?Siltuximab is a monoclonal antibody tar …16th Jan 2025 -
Sacituzumab: Exploring its Role in Cancer Research and the Rise of Biosimilars
Key Facts About SacituzumabWhat is Sacituzumab?Sacituzumab is an antibody-drug conjuga …16th Jan 2025