Sacituzumab: Exploring its Role in Cancer Research and the Rise of Biosimilars
Key Facts About Sacituzumab
What is Sacituzumab?
Sacituzumab is an antibody-drug conjugate (ADC) that targets the tumor-associated antigen Trop-2, used to treat various cancers, including breast and bladder cancers.
What is the mechanism of action for Sacituzumab?
Sacituzumab delivers a cytotoxic agent directly to tumor cells via its Trop-2 targeting, providing targeted therapy that reduces systemic toxicity.
What are the clinical applications of Sacituzumab?
Sacituzumab is used in treating advanced triple-negative breast cancer (TNBC) and urothelial carcinoma. Ongoing studies are exploring its efficacy in other cancers, including brain metastasis.
Is Sacituzumab safe?
While generally well-tolerated, Sacituzumab can cause side effects, including neutropenia, anemia, and gastrointestinal issues.
What role does Sacituzumab play in targeting Trop-2?
Sacituzumab targets Trop-2, a surface protein overexpressed in many cancers, delivering potent chemotherapy directly to tumor cells.
1.) Understanding Sacituzumab
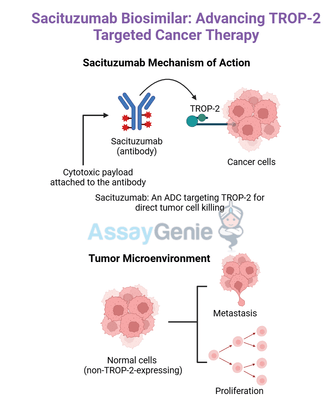
Sacituzumab is an innovative antibody-drug conjugate (ADC) that combines targeted immunotherapy with the precision of modern chemotherapy. This therapeutic agent is engineered by linking a monoclonal antibody specifically targeting Trop-2, a cell surface protein highly expressed in numerous cancers, to SN-38, an active metabolite of irinotecan and a potent topoisomerase I inhibitor. By leveraging this dual-action design, sacituzumab delivers chemotherapy directly to cancer cells with remarkable precision.
Trop-2 is overexpressed in various malignancies, including triple-negative breast cancer (TNBC), hormone-receptor-positive breast cancer, urothelial carcinoma, and some gastrointestinal cancers, making it an attractive therapeutic target. Upon binding to Trop-2 on the surface of tumor cells, sacituzumab is internalized, releasing the cytotoxic payload, SN-38, directly into the cancer cell. This mechanism allows for effective tumor cell death while sparing healthy tissues, leading to fewer systemic side effects compared to conventional chemotherapy.
The design of sacituzumab reflects the growing emphasis on precision oncology, aiming to maximize therapeutic efficacy while minimizing collateral damage. Its development represents a significant step forward in cancer treatment, offering new hope for patients with difficult-to-treat cancers, particularly those with limited options in advanced stages.
Prefer to Listen? Check out the Sacituzumab Podcast Episode
2.) Mechanism of Action of Sacituzumab
Sacituzumab's mechanism of action revolves around its ability to selectively target and kill cancer cells while sparing healthy tissues. The drug is an antibody-drug conjugate (ADC) that combines a monoclonal antibody specific to Trop-2, a protein overexpressed on the surface of many cancer cells, with SN-38, a potent chemotherapeutic agent derived from irinotecan.
Upon administration, sacituzumab binds to Trop-2 with high affinity. Trop-2 is not only abundant on cancer cells but also plays a role in tumor growth, making it an ideal target for therapeutic intervention. After binding, the drug-antibody complex is internalized into the cancer cell, where it undergoes degradation in lysosomes. This process releases SN-38 directly into the cancer cell, ensuring a highly localized and concentrated delivery of the chemotherapeutic agent.
SN-38 functions as a topoisomerase I inhibitor, disrupting DNA replication and leading to cell death, particularly in rapidly dividing tumor cells. This targeted approach significantly enhances the efficacy of chemotherapy while reducing systemic toxicity and side effects commonly associated with traditional chemotherapeutic regimens.
This innovative mechanism of action has positioned sacituzumab as a breakthrough in oncology, particularly in treating cancers with high Trop-2 expression, such as triple-negative breast cancer and urothelial carcinoma. Its precision delivery system exemplifies the advancements in targeted cancer therapies.

3.) Clinical Applications of Sacituzumab
Sacituzumab has emerged as a groundbreaking therapeutic option in oncology, particularly for patients with advanced and hard-to-treat cancers. Its most notable successes have been in the treatment of triple-negative breast cancer (TNBC) and urothelial carcinoma, two malignancies that often exhibit limited responses to traditional therapies. Sacituzumab’s approval for metastatic TNBC represents a significant advancement, as this subtype is known for its aggressive nature and poor prognosis.
In urothelial carcinoma, sacituzumab has shown promising efficacy in patients who have progressed after chemotherapy or immune checkpoint inhibitors. These results have expanded its role in addressing cancers with unmet medical needs.
Beyond its established indications, sacituzumab is being actively investigated in other malignancies, such as non-small cell lung cancer (NSCLC) and cancers associated with brain metastases. Its ability to deliver targeted chemotherapy makes it an attractive candidate for use in challenging metastatic environments, including the brain, where traditional therapies often face barriers to efficacy.
Recent studies have also explored the potential of sacituzumab in combination therapies, integrating it with immune checkpoint inhibitors or other targeted agents to enhance anti-cancer effects. Ongoing clinical trials are evaluating its application in earlier disease stages and across a broader spectrum of Trop-2-expressing cancers, further underscoring its transformative potential in oncology.
4.) Exploring Biosimilars for Sacituzumab
Biosimilars, like the Sacituzumab biosimilar, provide a valuable option for researchers seeking cost-effective alternatives in clinical research. These products are designed to closely mimic the reference drug, Sacituzumab, ensuring similar efficacy and safety profiles in laboratory settings.
What is a Biosimilar?
A biosimilar is a biologic product that is highly similar to an already approved reference biologic drug, with no significant differences in terms of safety, potency, and efficacy. Biosimilars help reduce research costs and make innovative therapies more accessible, particularly in the context of cancer research where novel treatments are often costly.
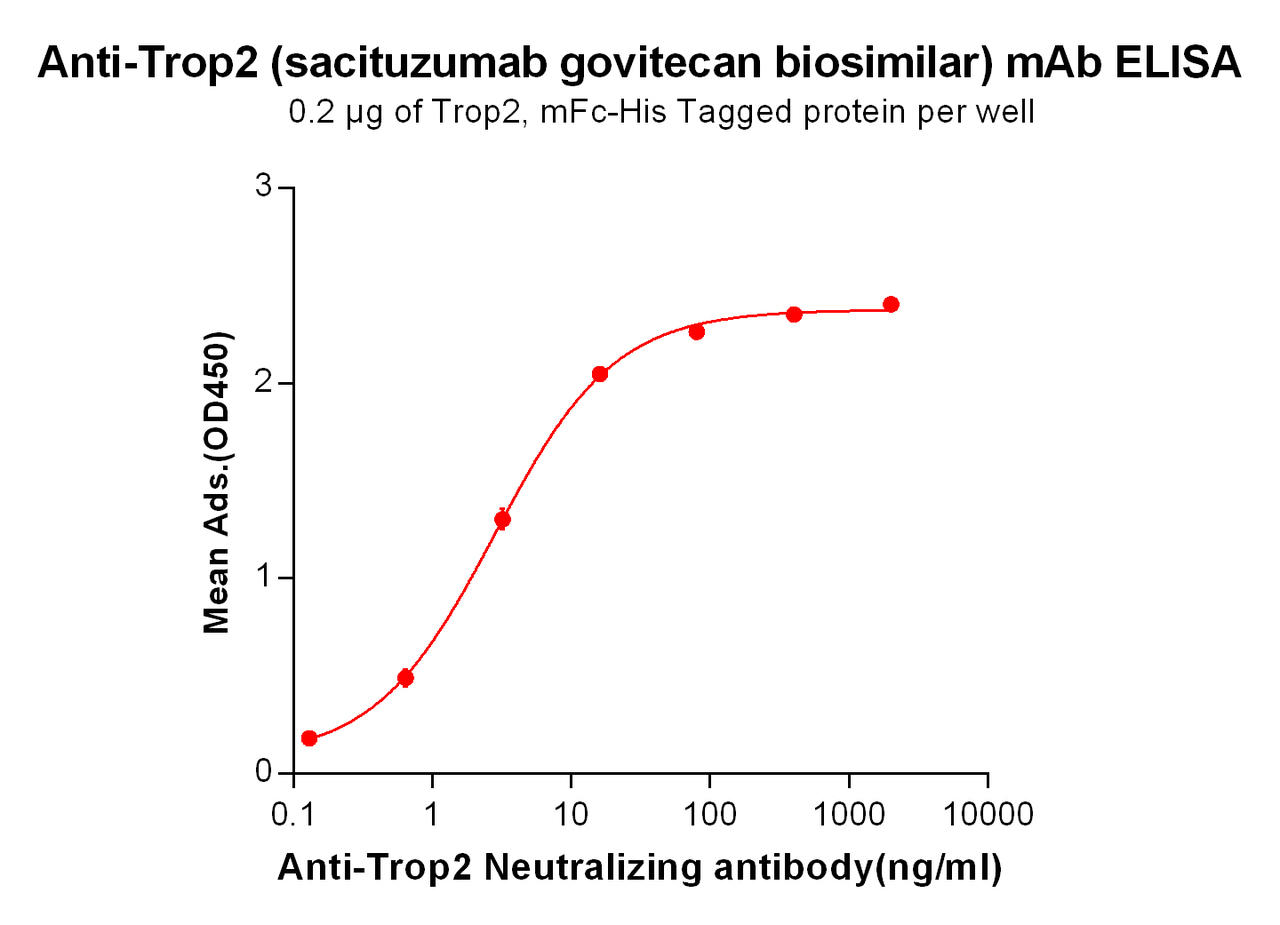
| Sacituzumab (Anti-Trop2) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | Trop2 |
| Reactivity: | Human |
Comparison
The Sacituzumab biosimilar closely resembles the reference drug Sacituzumab in terms of its mechanism of action, targeting Trop-2. However, as a research tool, the biosimilar allows for a more affordable alternative for experimental studies. Importantly, while it mimics the original drug's effects, it is not intended for clinical use in patients outside of controlled research environments.
Benefits
Research Use Only Disclaimer
The Sacituzumab biosimilar is intended for research use only and is not approved for human clinical use. Its role is to aid in advancing scientific discovery in cancer therapies and related research areas.
For more information, visit the Sacituzumab Biosimilar Product Page.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Marina Alberto, PhD
Marina Alberto, PhD, holds a robust academic background in Biotechnology, earning her Bachelor’s Degree and PhD in Science and Technology from Quilmes National University. Her research spans cancer immunotherapy, glycan profiling, and vaccine development, including innovative projects on pediatric leukemia diagnosis and cancer-associated carbohydrate-mimetic vaccines. She currently serves as a Technical Support and Sales Specialist at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025