TNFR2: Taming Regulatory T Cells to Enhance Anti-Tumor Immunity
In recent years, cancer immunotherapy has become a powerful tool for treating various malignancies. One promising approach involves targeting the tumor microenvironment, particularly regulatory T cells (Tregs), which play a key role in suppressing the immune response against tumors. TNFR2 (Tumor Necrosis Factor Receptor 2) is highly expressed on Tregs within the tumor, making it an attractive target for immunotherapy. Antibodies such as https://www.assaygenie.com/tnfr2-taming-regulatory-t-cells-to-enhance-anti-tumor-immunity/, which block TNFR2, have the potential to enhance anti-tumor immunity by reducing the suppressive function of Tregs and promoting a more robust immune response.
What is TNFR2?
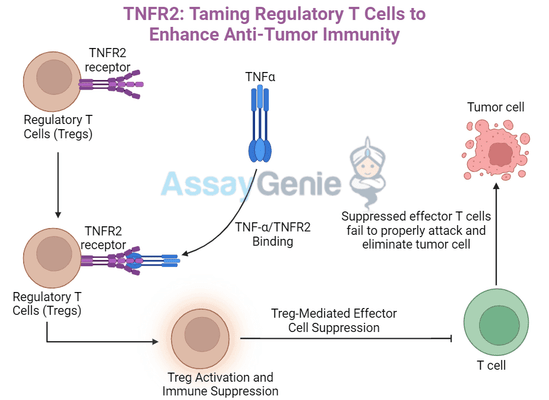
TNFR2, a member of the TNF receptor superfamily, is primarily expressed on Tregs, myeloid-derived suppressor cells (MDSCs), and some tumor cells. TNFR2 plays a critical role in the activation and proliferation of Tregs, which are key immune suppressors that help maintain immune tolerance. While Tregs are essential for preventing autoimmune reactions, they can also be exploited by tumors to evade immune destruction.
Tumors overexpress TNFR2 to expand Treg populations, creating an immunosuppressive environment that prevents the immune system from attacking cancer cells. As a result, targeting TNFR2 has become a novel strategy for disrupting this suppression and unleashing the immune system's anti-tumor capabilities.
Key Functions of TNFR2
Function | Role in Cancer |
|---|---|
Promotes Treg proliferation | Enhances immune suppression within the tumor microenvironment |
Activates immune-suppressive cells | Supports the growth of Tregs and MDSCs, dampening immune response |
Affects tumor cells directly | TNFR2 expression on some tumor cells promotes survival and growth |
Targeting TNFR2 in Cancer Immunotherapy
Given its role in promoting Treg-mediated immune suppression, TNFR2 represents an attractive target for cancer immunotherapy. By blocking TNFR2, the immune system can better overcome the suppressive environment created by tumors, allowing for a stronger and more effective anti-tumor response.
Anti-TNFR2 Antibodies: TR75-89
One of the most promising approaches to targeting TNFR2 is through the use of anti-TNFR2 antibodies, such as TR75-89. These antibodies are designed to block the interaction between TNFR2 and its ligands, preventing the activation and expansion of Tregs. This leads to a reduction in Treg numbers and function, which enhances the ability of effector T cells to attack and destroy tumor cells.
Mechanism of Action of TR75-89
- TNFR2 Blockade: TR75-89 binds to TNFR2, preventing its activation by TNF-α and other ligands.
- Reduction of Tregs: Blocking TNFR2 impairs Treg survival and proliferation, reducing their suppressive influence on the immune response.
- Increased Effector T Cell Activity: With Tregs diminished, effector T cells (such as CD8+ cytotoxic T cells) can more effectively attack the tumor.
- Tumor Reduction: Enhanced immune response leads to increased tumor cell destruction.
Therapeutic Agent | Target | Mechanism of Action | Effect on Tumor |
|---|---|---|---|
TR75-89 (Anti-TNFR2) | Blocks Treg activation and expansion | Reduces immune suppression, enhances T cell response | |
Prevents T-cell inhibition | Restores immune attack on tumors | ||
Anti-CTLA-4 | Blocks early immune checkpoint signaling | Amplifies T-cell activity |
The Role of TNFR2 in Tumor Microenvironment
The tumor microenvironment (TME) is rich in immune-suppressive cells, particularly Tregs, which express high levels of TNFR2. By engaging TNFR2, the tumor is able to foster a suppressive environment that protects cancer cells from immune attack. Targeting TNFR2 with therapies like TR75-89 can disrupt this protection, making it easier for immune cells to penetrate the TME and exert anti-tumor effects.
Combination Therapies with Anti-TNFR2
Anti-TNFR2 therapies like TR75-89 have shown potential for use in combination with other immune checkpoint inhibitors, such as anti-PD-1 or anti-CTLA-4 antibodies. These combinations can provide a multi-pronged approach to enhancing immune responses by both removing Treg suppression (via TNFR2 blockade) and directly activating effector T cells.
Cancer Type | Combination Strategy | Expected Outcome |
|---|---|---|
Anti-TNFR2 + Anti-PD-1 | Enhanced T-cell activation, increased tumor control | |
Non-small cell lung cancer (NSCLC) | Increased immune infiltration, improved survival | |
Synergistic tumor reduction, longer progression-free survival |
Challenges and Future Directions
While the early research on TNFR2-targeting therapies like TR75-89 is promising, several challenges remain:
- Patient Selection: Identifying patients who will benefit most from TNFR2-targeting therapies is crucial, as not all tumors express high levels of TNFR2.
- Immune-Related Toxicities: Like other immunotherapies, targeting TNFR2 may lead to immune-related adverse events (irAEs), such as inflammation or autoimmunity. Managing these side effects is essential for the success of the therapy.
- Combination Therapies: Determining the most effective combination strategies, such as pairing anti-TNFR2 with checkpoint inhibitors or chemotherapy, will be key to optimizing therapeutic outcomes.
Conclusion
TNFR2 is an exciting new target in the field of cancer immunotherapy, with the potential to modulate the tumor immune microenvironment by suppressing regulatory T cells. Antibodies like TR75-89 offer a promising approach to enhance anti-tumor immunity by reducing Treg-mediated suppression and allowing effector T cells to attack cancer more effectively. As research continues to explore the potential of anti-TNFR2 therapies, they may become a valuable addition to the arsenal of treatments designed to harness the immune system against cancer.
References
- Vanamee, É. S., & Faustman, D. L. (2017). TNFR2: A Novel Target for Cancer Immunotherapy. Trends in Molecular Medicine, 23(11), 1037-1046.
- Torrey, H., Butterworth, J., & Rosenblum, M. D. (2020). Targeting TNFR2 in Regulatory T Cells: A New Paradigm in Immunotherapy. Frontiers in Immunology, 11, 501.
- Yang, S., & Wang, X. (2021). TNFR2 as a Potential Target for Immunotherapy: Tumor Microenvironment and Immune Regulation. Nature Reviews Immunology, 21, 137-150.
- Chopra, M., & Schröder, J. (2016). TNFR2: A Molecule for Immune Modulation and Emerging Target for Cancer Therapy. International Journal of Cancer, 139(5), 1011-1020.
- Chen, X., & Oppenheim, J. J. (2011). TNF Receptor 2 and Its Role in Immunosuppression: A
Double-Edged Sword in Cancer Immunotherapy. Oncoimmunology, 10(8), 1081-1086. - Vanamee, É. S., & Faustman, D. L. (2018). The Role of TNFR2 in Autoimmune Diseases and Cancer. Immunological Reviews, 244(1), 12-23.
- Wang, J., & Zhang, M. (2021). Modulation of Tumor Immunity by Targeting TNFR2: Implications for Cancer Therapy. Cancer Letters, 502, 59-67.
- Oppenheim, J. J., & Yang, S. (2022). Understanding TNFR2: The Key to Unlocking Regulatory T Cell Therapy in Cancer. Journal of Clinical Investigation, 132(4), e150834.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025