Tiragolumab: Unlocking New Horizons in Cancer Immunotherapy
Quick Facts About Tiragolumab
What is Tiragolumab?
Tiragolumab is an anti-TIGIT monoclonal antibody developed to enhance immune responses against cancer cells. It is currently under investigation for its potential in treating various cancers
What is the mechanism of action for Tiragolumab?
Tiragolumab blocks TIGIT, a receptor on immune cells that inhibits anti-tumor activity, restoring the immune system's ability to attack cancer cells.
What cancers does Tiragolumab target?
Tiragolumab is being studied in non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), and other solid tumors, often in combination with atezolizumab.
What cancers does Tiragolumab target?
Preliminary studies indicate a manageable safety profile, with side effects similar to other immune checkpoint inhibitors, such as fatigue and immune-related adverse events.
1.) Understanding Tiragolumab
Tiragolumab, a groundbreaking immune checkpoint inhibitor developed by Genentech, a subsidiary of Roche, represents the next frontier in cancer immunotherapy. As an anti-TIGIT monoclonal antibody, Tiragolumab is designed to block TIGIT (T-cell immunoreceptor with Ig and ITIM domains), a protein that negatively regulates immune responses. TIGIT achieves this suppression by binding to its ligands, such as PVR, which are often overexpressed on tumor cells. This interaction suppresses the activation of T cells and natural killer (NK) cells, effectively dampening the immune system’s ability to recognize and destroy cancer cells. By inhibiting TIGIT, Tiragolumab reinvigorates these critical immune cells, enabling them to detect and eliminate tumor cells more effectively, thereby revitalizing the body’s natural defenses against cancer.
Tiragolumab is frequently administered in combination with atezolizumab, an anti-PD-L1 antibody, as part of a dual immune checkpoint blockade strategy. This combination targets two distinct yet complementary pathways of immune suppression in the tumor microenvironment. While atezolizumab blocks the PD-L1/PD-1 interaction that impairs T-cell function, Tiragolumab disrupts TIGIT-mediated suppression, providing a synergistic effect that amplifies anti-tumor immune responses. This approach has shown promise in clinical trials, particularly the CITYSCAPE study, which reported improved response rates in patients with high PD-L1 expression. High PD-L1 expression serves as a biomarker associated with better therapeutic outcomes, underscoring the potential for personalized treatment strategies.
Ongoing research is exploring Tiragolumab’s efficacy across various cancers, expanding its potential applications. With its ability to enhance immune activity and complement existing therapies, Tiragolumab is paving the way for more personalized, effective, and durable cancer treatment options, marking a significant advance in immunotherapy.
2.) Mechanism of Action of Tiragolumab
Tiragolumab's mechanism of action revolves around its ability to inhibit TIGIT (T-cell immunoreceptor with immunoglobulin and ITIM domains) signaling, which plays a crucial role in immune evasion by tumors. TIGIT is a checkpoint receptor primarily expressed on T cells and NK cells. Under normal conditions, TIGIT binds to its ligands, such as CD155 and CD112, which are overexpressed on tumor cells. This binding suppresses the activation and function of T cells and NK cells, thus enabling tumors to evade immune detection and destruction.
By blocking this interaction, Tiragolumab effectively restores the functionality of immune cells in the tumor microenvironment. One of the key outcomes is the revitalization of exhausted T cells, which are often found in the presence of tumors. These exhausted T cells, which have previously failed to mount an effective immune response, are reinvigorated, allowing them to better recognize and target tumor cells for destruction. This is particularly important in cancers where T-cell exhaustion is a barrier to effective treatment.
In addition to restoring T-cell function, Tiragolumab enhances the activity of NK cells, a critical component of the innate immune system. By boosting NK cell-mediated cytotoxicity, Tiragolumab helps increase the immune system's ability to recognize and kill tumor cells, even those that have been able to evade detection by other immune mechanisms.
Moreover, Tiragolumab synergizes with PD-L1 blockade therapies, such as atezolizumab. This combination therapy helps overcome multiple immune evasion strategies utilized by tumors, further enhancing the immune response against cancer. Tiragolumab's dual mechanism of action is particularly effective in "cold tumors," which typically show low levels of immune cell infiltration and are resistant to traditional therapies. By transforming these "cold" tumors into "hot" tumors, Tiragolumab holds significant potential in treating cancers that were previously unresponsive to immune-based therapies. This transformative effect is opening up new therapeutic avenues for patients with such refractory cancers.
3.) Clinical Applications of Tiragolumab
Tiragolumab is undergoing extensive clinical evaluation across multiple cancer types, with significant progress being made, particularly in lung cancer treatments.
Non-Small Cell Lung Cancer (NSCLC): In Non-Small Cell Lung Cancer (NSCLC), one of the most common and aggressive forms of lung cancer, Tiragolumab has shown promising results, especially when combined with the PD-L1 inhibitor atezolizumab. The CITYSCAPE trial, a pivotal phase II study, demonstrated that this combination therapy significantly improved progression-free survival (PFS) and overall response rates (ORR) in patients with PD-L1-high NSCLC. These findings are especially important because NSCLC patients with high PD-L1 expression tend to have poorer clinical outcomes. By enhancing immune activation in these patients, Tiragolumab combined with atezolizumab may represent a breakthrough in improving treatment effectiveness.
Small Cell Lung Cancer (SCLC): In Small Cell Lung Cancer (SCLC), an aggressive and challenging cancer type, early-phase studies are investigating the efficacy of Tiragolumab. SCLC is known for its rapid progression and resistance to conventional treatments, leaving patients with limited therapeutic options. Early data from ongoing trials suggest that Tiragolumab may provide a new avenue for enhancing immune responses in SCLC patients and overcoming the immune evasion mechanisms employed by tumors in this aggressive cancer type.
Other Solid Tumors: Tiragolumab is also being explored in a range of other solid tumors. Clinical trials are currently assessing its potential in cancers such as melanoma, cervical cancer, and esophageal cancer. These cancers often exhibit significant immune resistance, making them difficult to treat with traditional therapies. Tiragolumab’s mechanism of enhancing immune cell activation could offer a promising new therapeutic approach in overcoming immune evasion and improving treatment outcomes.
Future Prospects and Biomarker Development: The encouraging results from these studies have positioned Tiragolumab as a potential game-changer in oncology. Ongoing and future clinical trials will aim to confirm its therapeutic benefits across a broader range of patient populations and cancer types. Additionally, research will focus on refining biomarkers to predict which patients are most likely to respond to Tiragolumab, further enhancing its precision in cancer treatment. These efforts will help optimize patient outcomes and establish Tiragolumab as a groundbreaking immuno-oncology therapy.
4.) Exploring Biosimilars for Tiragolumab
What is a Biosimilar?
Biosimilars are highly similar copies of biologic drugs, designed to provide comparable safety and efficacy. Unlike generic drugs, biosimilars are produced using living cells, making them complex and challenging to manufacture.
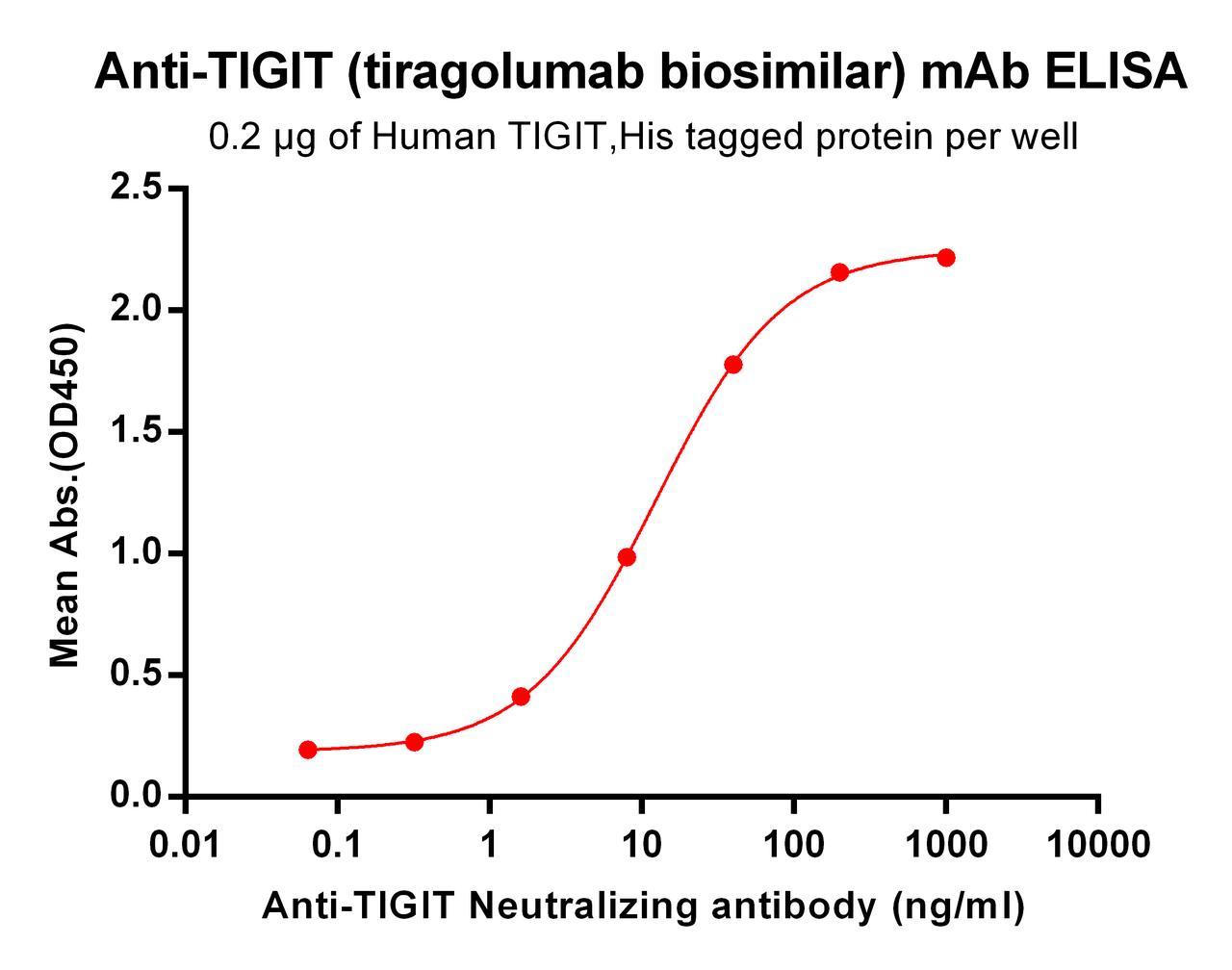
| Tiragolumab (Anti-TIGIT) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | TIGIT |
| Reactivity: | Human |
How Tiragolumab Biosimilars Advance Research
Biosimilars for Tiragolumab are emerging as invaluable tools for research purposes. They enable scientists to:
Expand Preclinical Studies: Investigate Tiragolumab’s mechanism and potential new combinations.
Reduce Research Costs: Offer cost-effective alternatives for academic and industrial studies.
Foster Innovation: Provide access to cutting-edge immunotherapy tools for developing next-generation treatments.
Key Differences and Benefits
While Tiragolumab and its biosimilars share the same target—TIGIT—biosimilars are designated "for research use only" and are not approved for clinical applications. Their primary value lies in supporting discovery and expanding access to high-quality research materials.
Research Use Only Disclaimer:
Tiragolumab biosimilars are intended for research use only and are not approved for clinical or therapeutic purposes.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Miren Ruiz de Eguilaz, PhD
Miren Ruiz de Eguilaz, PhD, has an extensive academic background, earning a BSc in Biology from UPV/EHU, an MSc in Biotechnology from the University of Oviedo, and a PhD in Chemistry from Dublin City University (DCU). Miren’s expertise lies in biosensor technology and bacterial diagnostics. She currently serves as a Product Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Ivuxolimab: Redefining Cancer Immunotherapy and Research
Quick Facts About IvuxolimabWhat is ivuxolimab?Ivuxolimab is a monoclonal antibody tar …1st Feb 2025



