SIRPα Inhibition: Clearing Tumors by Promoting Phagocytosis
Cancer cells are adept at avoiding destruction by the immune system, often exploiting specific pathways to escape detection and elimination. One of these key mechanisms involves the SIRPα-CD47 axis, which prevents macrophages and other phagocytic cells from attacking tumor cells. SIRPα inhibitors, such as P84, are emerging as novel immunotherapies that block this protective signal, allowing the immune system to recognize and engulf cancer cells. This article explores the role of SIRPα inhibition in enhancing phagocytosis, promoting anti-tumor immunity, and the therapeutic potential of anti-SIRPα agents like P84 in cancer treatment.
The Role of SIRPα in Immune Regulation
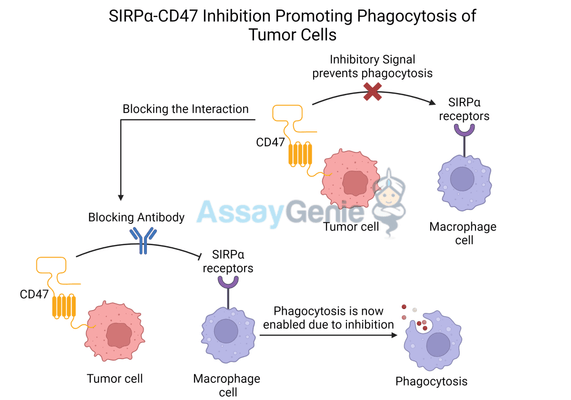
Signal regulatory protein alpha (SIRPα) is a transmembrane receptor primarily expressed on myeloid cells, including macrophages, dendritic cells, and neutrophils. It functions as a key inhibitory receptor, interacting with CD47, a molecule expressed on the surface of both normal and cancer cells. This SIRPα-CD47 interaction sends a “don’t eat me” signal, effectively preventing macrophages from engulfing and destroying the cell.
Mechanism of Tumor Immune Evasion
Tumor cells often overexpress CD47, which binds to SIRPα on macrophages, sending inhibitory signals that prevent phagocytosis, the process by which immune cells engulf and digest harmful pathogens or cellular debris. This immune evasion allows tumors to proliferate unchecked, shielded from one of the immune system's primary defenses.
By inhibiting SIRPα, this protective signaling is disrupted, enabling macrophages and other immune cells to target and eliminate tumor cells more effectively. SIRPα blockade thus serves as a key strategy to overcome tumor immune evasion.
Mechanism | Effect on Tumor |
|---|---|
SIRPα-CD47 interaction | Inhibits macrophage-mediated phagocytosis of tumor cells. |
SIRPα inhibition (e.g., P84) | Promotes macrophage activation and tumor clearance |
Anti-SIRPα Therapies: Promoting Tumor Clearance
SIRPα inhibitors, such as P84, work by blocking the CD47-SIRPα axis, thereby promoting the phagocytosis of tumor cells. These therapies reawaken the natural ability of macrophages to engulf and destroy cancer cells, which can otherwise evade the immune system by upregulating CD47.
Mechanism of Action: How Anti-SIRPα Antibodies Work
Anti-SIRPα antibodies like P84 bind to the SIRPα receptor on macrophages, preventing its interaction with CD47 on tumor cells. This blockade effectively removes the inhibitory signal, allowing macrophages to perform phagocytosis and eliminate cancer cells. The result is an enhanced innate immune response, which can also improve the overall anti-tumor immunity by increasing antigen presentation to T cells.
Anti-SIRPα Effect | Outcome |
|---|---|
Blocking SIRPα-CD47 interaction | Restores macrophage ability to engulf tumor cells |
Enhanced phagocytosis | Promotes tumor destruction and immune clearance |
Increased antigen presentation | Boosts adaptive immune responses by T cells |
P84: A Promising Anti-SIRPα Antibody
Among the anti-SIRPα antibodies, P84 has shown significant potential in preclinical cancer models. By inhibiting SIRPα, P84 enables macrophages to bypass the tumor's defense mechanism and perform effective phagocytosis. This leads to tumor regression and improves the efficacy of other cancer therapies.
Enhancing Tumor Clearance
P84 has been shown to enhance the body's ability to recognize and destroy tumor cells by shifting the balance in favor of pro-phagocytic signaling. In preclinical studies, the use of P84 has led to increased tumor infiltration by macrophages, higher rates of tumor cell engulfment, and an overall boost in anti-tumor activity.
Synergy with Other Cancer Immunotherapies
SIRPα inhibition is particularly promising when used in combination with other immunotherapies. For example, combining P84 with checkpoint inhibitors (such as PD-1 or CTLA-4 inhibitors can further enhance the immune response against tumors. While checkpoint inhibitors remove the brakes on T cells, SIRPα inhibition boosts macrophage activity, leading to a multi-pronged attack on cancer cells.
Combination Therapy | Benefit |
|---|---|
Anti-SIRPα + checkpoint inhibitors | Enhances both innate (macrophage) and adaptive (T cell) immune responses |
Anti-SIRPα + anti-CD47 | Double blockade of “don’t eat me” signal, amplifying macrophage activity |
Ongoing Clinical Trials
Several clinical trials are evaluating the effectiveness of anti-SIRPα therapies in various cancer types, including hematologic malignancies and solid tumors. These trials aim to determine the safety, dosing, and therapeutic potential of anti-SIRPα agents like P84, both as monotherapy and in combination with other immunotherapies.
Clinical Trial | Cancer Type | Combination Therapy | Phase |
|---|---|---|---|
NCT03990233 | Non-Hodgkin lymphoma | Anti-SIRPα + anti-CD47 | Phase I |
NCT04028762 | Solid tumors | Anti-SIRPα + checkpoint inhibitors | Phase II |
NCT05189093 | Acute myeloid leukemia | Anti-SIRPα + chemotherapy | Phase I |
Macrophage Reprogramming: From Tumor Support to Tumor Destruction
One of the key advantages of SIRPα inhibition is its ability to reprogram macrophages in the tumor microenvironment. Normally, many tumors are surrounded by tumor-associated macrophages (TAMs) that exist in an M2 state—a pro-tumor phenotype that promotes tumor growth and suppresses immune responses. By inhibiting SIRPα, macrophages are shifted to an M1 phenotype, which is anti-tumor and actively promotes tumor cell clearance.
Macrophage Phenotype | Function |
|---|---|
M2 (Pro-tumor) | Promotes tumor growth, suppresses immune response |
M1 (Anti-tumor) | Engages in phagocytosis, promotes immune-mediated tumor clearance |
Advantages and Challenges of SIRPα Inhibition
While anti-SIRPα therapies like P84 hold great promise, there are several challenges and considerations to address:
Advantages of SIRPα Inhibition
- Restoration of immune surveillance: By blocking the CD47-SIRPα axis, these therapies re-engage macrophages in tumor destruction.
- Synergy with other therapies: Anti-SIRPα antibodies work well in combination with checkpoint inhibitors and other immune-based therapies, enhancing the overall anti-tumor response.
- Broad applicability: The SIRPα-CD47 axis is a common mechanism used by many tumor types, making SIRPα inhibition potentially effective across a wide range of cancers.
Challenges
- Off-target effects:
CD47 is expressed on normal cells as well, which can lead to off-target effects like anemia due to macrophage-mediated destruction of red blood cells. However, targeting SIRPα specifically on macrophages offers a more focused therapeutic approach. - Tumor heterogeneity:
Not all tumors express high levels of CD47 or rely on the SIRPα-CD47 axis for immune evasion, limiting the efficacy of anti-SIRPα therapies in some cancers.
Future Directions for SIRPα Inhibition
The future of SIRPα inhibition lies in further refining these therapies and identifying the patient populations and cancer types that will benefit most. Researchers are exploring how to enhance the specificity of these agents, reduce off-target effects, and optimize combination strategies with other immunotherapies.
As clinical trials progress, SIRPα inhibitors may soon become a critical part of the cancer immunotherapy landscape, offering new hope for patients with refractory tumors that have resisted other forms of treatment.
Conclusion
SIRPα inhibition, through agents like P84, represents a promising new approach to cancer immunotherapy by restoring the phagocytic activity of macrophages and enhancing the immune system’s ability to clear tumors. By targeting the SIRPα-CD47 axis, these therapies overcome a key mechanism of tumor immune evasion, allowing for more effective tumor destruction. As research and clinical trials continue, SIRPα inhibition holds the potential to revolutionize cancer treatment, especially when combined with other immune-based therapies.
References
- Barclay, A.N., 2009. Signal regulatory protein α (SIRPα): a multi-domain phagocyte signaling receptor. Immunological Reviews, 234(1), pp.75-85.
- Weiskopf, K., et al., 2013. SIRPα blockade enhances macrophage-mediated clearance of cancer cells in xenograft models of
hematologic malignancies. Blood, 121(26), pp.4906-4914. - Liu, J., et al., 2015. Preclinical development of a humanized anti-SIRPα antibody with anti-cancer therapeutic potential. Proceedings of the National Academy of Sciences, 112(40), pp.12067-12072.
- Tsai, R.K. and Discher, D.E., 2008. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. The Journal of Cell Biology, 180(5), pp.989-1003.
- Huang, Y., et al., 2020. Targeting CD47/SIRPα for cancer immunotherapy: clinical progress and challenges. Frontiers in Immunology, 11, p.18.
- Chao, M.P., et al., 2010. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Research, 70(4), pp.1234-1243.
- Alvey, C., et al., 2019. Tumor-associated macrophages in cancer: good guys or bad guys? The Journal of Immunology, 202(6), pp.1903-1912.
- Wu, L., et al., 2017. Blockade of CD47/SIRPα signaling enhances tumor antigen presentation and synergizes with T-cell checkpoint blockade to promote anti-tumor responses in glioblastoma. Nature Communications, 8(1), p.16085.
Recent Posts
-
Metabolic Exhaustion: How Mitochondrial Dysfunction Sabotages CAR-T Cell Therapy in Solid Tumors
Imagine engineering a patient's own immune cells into precision-guided missiles against cancer—cells …8th Dec 2025 -
The Powerhouse of Immunity: How Mitochondrial Fitness Fuels the Fight Against Cancer
Why do powerful cancer immunotherapies work wonders for some patients but fail for others? The answe …5th Dec 2025 -
How Cancer Cells Hijack Immune Defenses Through Mitochondrial Transfer
Imagine a battlefield where the enemy doesn't just hide from soldiers—it actively sabotages their we …5th Dec 2025