Etigilimab: Unveiling the Role of Anti-TIGIT in Cancer Research
Key Facts About Etigilimab
What is Etigilimab?
Etigilimab is an anti-TIGIT monoclonal antibody being studied for its potential role in cancer immunotherapy.
What role does Etigilimab play in targeting TIGIT?
Etigilimab inhibits TIGIT, a receptor that suppresses T-cell activity, enhancing the immune system's ability to combat tumors.
What are the clinical applications of Etigilimab?
Etigilimab is being explored in combination therapies for treating various cancers, leveraging its immune-boosting capabilities.
1.) Understanding Etigilimab
Etigilimab, also known by its research name MGA271, is a monoclonal antibody that targets TIGIT (T-cell immunoreceptor with Ig and ITIM domains). TIGIT is an immune checkpoint receptor that dampens immune responses, allowing cancer cells to evade detection. By blocking TIGIT, Etigilimab reactivates T-cells and NK cells, fostering a robust anti-tumor immune response.
First developed by Mereo BioPharma, Etigilimab is part of the broader movement in cancer research to harness the immune system against malignancies. Anti-TIGIT therapies like Etigilimab are often investigated in combination with PD-1 or PD-L1 inhibitors to achieve synergistic effects. This dual blockade approach has shown promise in preclinical and early clinical trials.
TIGIT plays a pivotal role in the tumor microenvironment by binding to its ligands, CD155 and CD112, on tumor cells and antigen-presenting cells. This interaction suppresses the activation of T-cells and natural killer (NK) cells, dampening the immune response. By targeting TIGIT, Etigilimab helps restore the immune system's ability to recognize and destroy tumor cells effectively. The antibody’s design ensures high specificity and potency, making it a critical tool in advancing cancer immunotherapy.
Prefer to Listen? Check out the Etigilimab Podcast Episode
2.) Mechanism of Action of Etigilimab
Etigilimab’s mechanism revolves around its ability to block TIGIT’s interaction with its ligands, CD155 (PVR) and CD112 (nectin-2). These ligands are commonly expressed on tumor cells and antigen-presenting cells within the tumor microenvironment. When TIGIT binds to these ligands, it sends inhibitory signals that reduce T-cell activation and proliferation.
By inhibiting TIGIT, Etigilimab promotes T-cell activation, enhances cytokine production, and restores immune surveillance. Additionally, it boosts NK cell-mediated cytotoxicity, further contributing to its anti-cancer effects.
One of the key aspects of Etigilimab’s mechanism is its ability to disrupt the suppressive signaling cascade that TIGIT initiates. TIGIT competes with CD226 (DNAM-1), a co-stimulatory receptor, for binding to CD155 and CD112. In the presence of TIGIT, CD226’s activation is significantly diminished, leading to impaired immune responses. Etigilimab restores CD226-mediated signaling by preventing TIGIT’s inhibitory binding, thereby enhancing the activation and function of effector T-cells and NK cells.
Preclinical models have demonstrated that Etigilimab not only improves T-cell proliferation but also increases the production of key cytokines like interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α), which are critical for anti-tumor immunity. Furthermore, Etigilimab’s effects on NK cells amplify their ability to lyse tumor cells, providing a dual mechanism of immune activation.
In combination with PD-1/PD-L1 inhibitors, Etigilimab’s action is further potentiated. PD-1 blockade prevents T-cell exhaustion, while TIGIT inhibition restores T-cell and NK cell activity. This complementary interaction addresses multiple pathways of immune suppression, making it a promising strategy in cancer immunotherapy.
3.) Clinical Applications of Etigilimab
Etigilimab is being investigated for its role in treating various solid tumors, including non-small cell lung cancer (NSCLC), melanoma, and head and neck squamous cell carcinoma (HNSCC). Emerging studies suggest that Etigilimab may be particularly effective in combination therapies, where it complements the effects of existing immune checkpoint inhibitors.
Recent research highlights the importance of TIGIT as a therapeutic target due to its role in immune evasion. TIGIT is often upregulated in tumors, leading to suppressed T-cell activity and immune exhaustion. Etigilimab’s mechanism of action counters this process, reinvigorating immune cells and enhancing anti-tumor activity.
Early-phase clinical trials have shown encouraging signs of efficacy, particularly in cancers resistant to standard treatments. For instance, combinations of Etigilimab with PD-1 inhibitors like nivolumab have demonstrated potential in overcoming immune resistance. Additionally, ongoing research explores its applications in hematological malignancies and rare cancer types, broadening its therapeutic scope.
Despite challenges in clinical development, such as patient variability and trial limitations, Etigilimab remains a promising candidate in the evolving landscape of immuno-oncology. Its ability to target a novel checkpoint pathway offers hope for improving outcomes in cancers where traditional therapies fall short.

4.) Advancing Research on Etigilimab with Biosimilars
What is a Biosimilar?
A biosimilar is a highly similar and functionally equivalent version of a reference biologic drug. In research, biosimilars offer a cost-effective and accessible alternative for studying the mechanisms and applications of biologic therapies like Etigilimab.
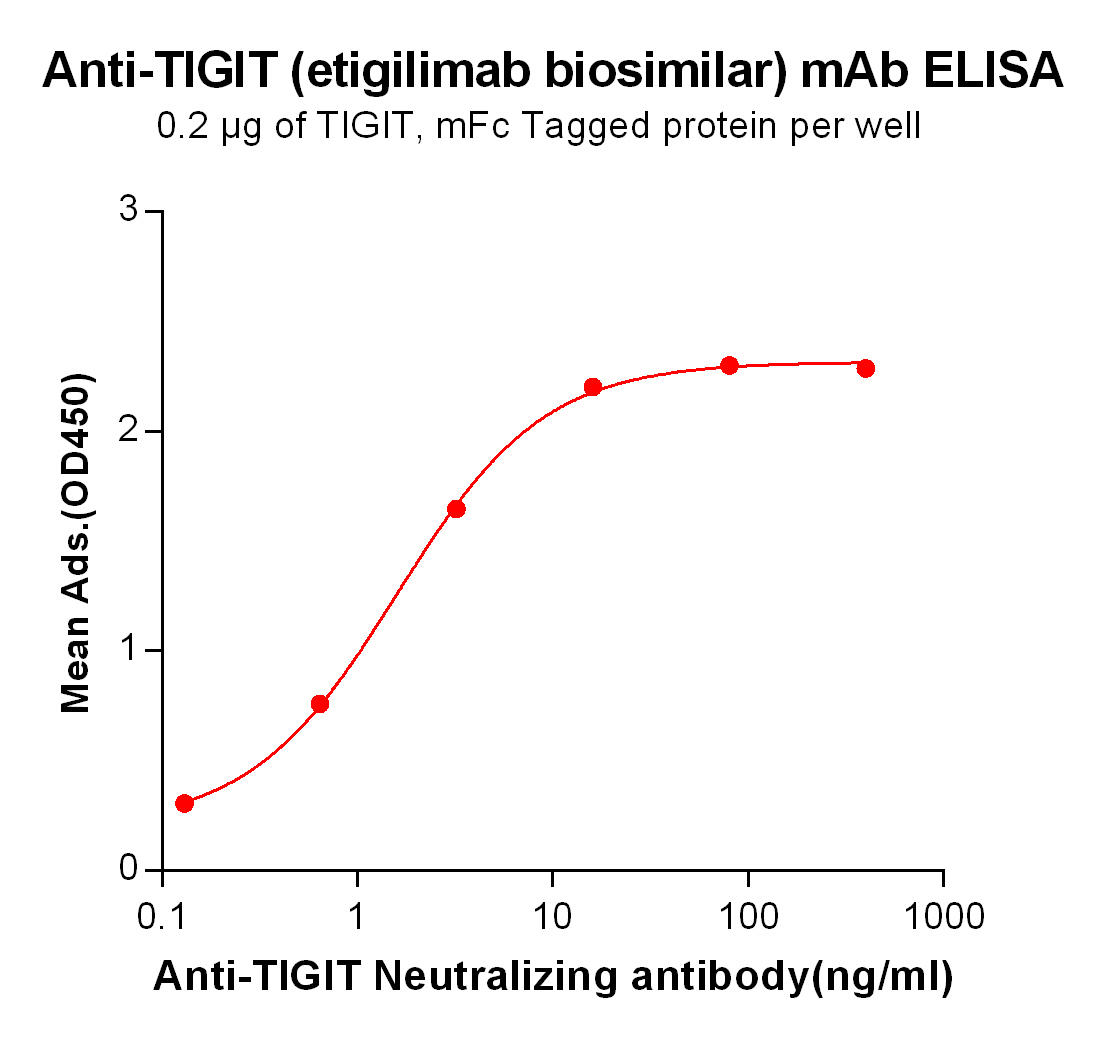
| Etigilimab (Anti-TIGIT) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | TIGIT |
| Reactivity: | Human |
How Does Etigilimab Biosimilar Compare?
Our Etigilimab biosimilar mirrors the structure and function of the original antibody, making it an invaluable tool for preclinical studies. Researchers can use this biosimilar to investigate TIGIT’s role, optimize combination therapies, and explore new applications of checkpoint inhibition without the high costs associated with the original biologic.
Benefits of Using Biosimilars in Research
- Cost-Effectiveness: Biosimilars provide a more affordable option for conducting extensive studies.
- Accessibility: They ensure broader availability to researchers worldwide.
- Consistency: With rigorous manufacturing standards, biosimilars deliver reliable and reproducible results.
Research Use Only Disclaimer:
Please note that our Etigilimab biosimilar is intended for research use only and not for clinical or patient applications.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By David Lee, PhD
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025




