Zolbetuximab: A Breakthrough in Targeting Claudin 18.2 for Cancer Treatment
Quick Facts About Zolbetuximab
What is Zolbetuximab?
Zolbetuximab is a monoclonal antibody that targets Claudin 18.2, a protein overexpressed in gastric and pancreatic cancers, enhancing targeted therapy.
What is the mechanism of action for Zolbetuximab?
It binds to Claudin 18.2, triggering antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), leading to tumor cell death.
What are the clinical applications of Zolbetuximab?
It is being investigated for treating gastric and pancreatic cancers, particularly in patients with high Claudin 18.2 expression.
1.) Understanding Zolbetuximab
Zolbetuximab is an investigational monoclonal antibody developed by Astellas Pharma, designed to target Claudin 18.2, a tight junction protein found in certain cancer cells. Claudin 18.2 is normally restricted to gastric epithelial cells, but in malignancies such as gastric and pancreatic cancer, its expression becomes aberrant, making it a highly specific target for therapeutic intervention. This selectivity allows Zolbetuximab to differentiate between cancerous and normal cells, reducing the likelihood of off-target toxicity commonly associated with conventional chemotherapy.
The interest in Claudin 18.2 as a target for precision oncology has grown due to its role in tumor progression and metastasis. Research indicates that Claudin 18.2 overexpression is found in approximately 30–50% of gastric cancer cases and a subset of pancreatic cancers, offering a significant therapeutic window. Patients with high Claudin 18.2 expression are believed to be more responsive to Zolbetuximab therapy, as the drug specifically binds to this biomarker, enhancing the immune system's ability to attack cancer cells.
Unlike traditional chemotherapeutic agents that indiscriminately affect rapidly dividing cells, Zolbetuximab leverages immune-mediated mechanisms to target cancer cells more precisely. This approach has the potential to improve patient outcomes by enhancing efficacy while minimizing systemic toxicity. As research progresses, Zolbetuximab’s role in precision medicine continues to expand, offering new hope for patients with Claudin 18.2-positive tumors. Clinical trials are underway to further evaluate its effectiveness and safety, with promising preliminary data positioning it as a valuable addition to the evolving landscape of targeted cancer therapies.
2.) Mechanism of Action of Zolbetuximab
Zolbetuximab exerts its anti-tumor effects through a targeted mechanism that selectively binds to Claudin 18.2, triggering multiple immune-mediated responses that result in cancer cell destruction. This precision-based approach distinguishes it from traditional chemotherapy, which often leads to widespread toxicity due to non-specific targeting of rapidly dividing cells.
One of Zolbetuximab’s primary mechanisms is Antibody-Dependent Cellular Cytotoxicity (ADCC). Once bound to Claudin 18.2-expressing tumor cells, the drug recruits immune effector cells, such as natural killer (NK) cells and macrophages. These immune cells recognize the Fc region of Zolbetuximab and release cytotoxic granules containing perforin and granzymes, which induce apoptosis in the tumor cells. This mechanism enhances the body's natural immune response to cancer, improving the specificity and effectiveness of the treatment.
In addition to ADCC, Zolbetuximab also triggers Complement-Dependent Cytotoxicity (CDC). Upon binding to tumor cells, it activates the complement cascade, leading to the formation of membrane attack complexes (MACs) that create pores in the cancer cell membrane. This results in direct lysis of the tumor cells, further enhancing the drug's cytotoxic potential.
Beyond immune-mediated killing, Zolbetuximab is believed to inhibit cellular proliferation by interfering with Claudin 18.2’s role in cell adhesion and signaling. By disrupting tumor cell cohesion, it may prevent cancer progression and metastasis, making it a multifaceted therapeutic approach.
This combination of ADCC, CDC, and proliferation inhibition ensures that Zolbetuximab maximizes tumor destruction while minimizing systemic side effects, reinforcing its potential as a game-changing agent in precision oncology.
3.) Clinical Applications of Zolbetuximab
Zolbetuximab is currently being evaluated in several clinical trials to assess its efficacy in treating cancers that express Claudin 18.2. As a precision oncology therapy, it holds potential for improving outcomes in patients with gastric and pancreatic cancers by specifically targeting tumor cells while minimizing systemic toxicity.
Gastric Cancer
Gastric cancer remains a significant global health burden, with limited targeted treatment options. Zolbetuximab is being investigated in the SPOTLIGHT Trial, a Phase III study designed to evaluate its effectiveness in combination with chemotherapy for patients with advanced gastric or gastroesophageal junction (GEJ) cancer. The trial focuses on individuals with high Claudin 18.2 expression, assessing whether Zolbetuximab improves progression-free survival and overall response rates compared to chemotherapy alone. Preliminary results suggest promising anti-tumor activity, reinforcing its potential role in front-line therapy.
Similarly, the GLOW Trial is another Phase III study examining Zolbetuximab as a first-line treatment for HER2-negative gastric cancer. HER2-negative patients currently have fewer targeted therapy options, making this trial particularly significant. Researchers aim to determine whether adding Zolbetuximab to standard chemotherapy enhances clinical benefits while maintaining a manageable toxicity profile.
Pancreatic Cancer
While research on Zolbetuximab for pancreatic cancer is in earlier stages, preliminary findings indicate that a subset of pancreatic tumors express Claudin 18.2. Ongoing studies are exploring its potential efficacy, particularly in combination with other treatments, to improve survival rates in this aggressive cancer type.
As these trials progress, Zolbetuximab may redefine treatment paradigms for Claudin 18.2-positive cancers.
4.) Exploring Biosimilars for Zolbetuximab
What is a Biosimilar?
Biosimilars are biologic drugs highly similar to original reference biologics, offering cost-effective alternatives without compromising efficacy. They play a crucial role in expanding research opportunities and accessibility to targeted therapies.
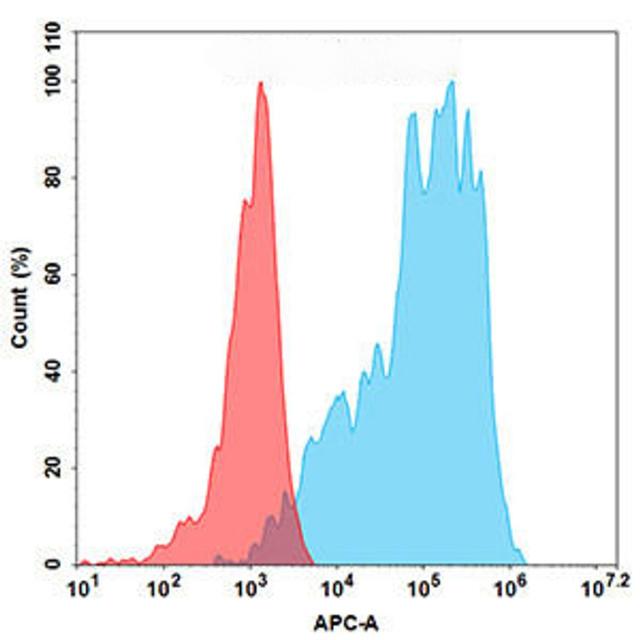
| Zolbetuximab (Anti-CLDN18.2) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | CLDN18.2 |
| Reactivity: | Human |
How Zolbetuximab Biosimilar Compares to Zolbetuximab
A biosimilar version of Zolbetuximab retains the reference drug’s mechanism of action, targeting Claudin 18.2 for cancer treatment. However, biosimilars are developed through independent manufacturing processes, ensuring affordability while maintaining therapeutic potential.
Benefits of Zolbetuximab Biosimilar in Research
- Cost-Effective Studies: Enables broader preclinical and translational research on Claudin 18.2 targeting.
- Increased Accessibility: Expands research applications in oncology labs worldwide.
- Regulatory and Quality Assurance: Meets stringent research-grade standards for non-clinical studies.
Research Use Only Disclaimer:
Zolbetuximab biosimilar is intended for research purposes only and is not approved for clinical use.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By David Lee, PhD
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



