Zilovertamab: Unlocking the Potential of ROR1-Targeted Therapy
Quick Facts About Zilovertamab
What is Zilovertamab?
Zilovertamab is a monoclonal antibody targeting ROR1, a receptor tyrosine kinase overexpressed in hematologic cancers and solid tumors.
How does Zilovertamab work?
It binds to ROR1, disrupting tumor cell survival pathways and enhancing immune-mediated cancer cell destruction.
What are the clinical applications of Zilovertamab?
It has been investigated for treating chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), and other malignancies.
Are there biosimilars for Zilovertamab?
Yes, biosimilars are being explored to enhance research accessibility and further therapeutic advancements.
1.) Understanding Zilovertamab
Zilovertamab is an innovative cancer immunotherapy that represents a promising approach in the treatment of various cancers by targeting ROR1 (Receptor tyrosine kinase-like orphan receptor 1), a protein that is highly expressed in a range of hematologic and solid tumors but is absent in normal adult tissues. ROR1’s role in regulating critical survival and proliferation signals in tumor cells makes it a compelling and specific therapeutic target. Unlike other cancer targets, ROR1 is not present in healthy adult tissues, which significantly reduces the risk of toxicity to normal cells.
The monoclonal antibody Zilovertamab works by selectively binding to ROR1 on the surface of cancer cells. This binding interrupts the signaling pathways that tumors rely on for survival, growth, and resistance to treatment. By inhibiting these pathways, Zilovertamab can effectively disrupt the tumor’s ability to proliferate and sustain itself, leading to a reduction in tumor mass and, in many cases, tumor cell death. Furthermore, this targeted approach minimizes the potential damage to surrounding healthy tissues, which is a common limitation of conventional chemotherapy.
Preclinical studies have demonstrated that Zilovertamab can induce apoptosis (programmed cell death) in a variety of cancer cell types. The efficacy of Zilovertamab in combination with other therapies, such as chemotherapy and immunotherapy, is being explored in clinical trials. These combinations aim to enhance its therapeutic effects, extend its clinical applications, and address cancers that currently have limited treatment options. Ongoing research is focused on identifying the most effective dosing strategies and understanding how Zilovertamab can work synergistically with other cancer treatments to improve overall patient outcomes.
2.) Mechanism of Action of Zilovertamab
Zilovertamab exerts its anti-cancer effects through a carefully orchestrated mechanism of action that focuses on inhibiting ROR1-mediated survival signals, which are vital for cancer cell maintenance. ROR1, a receptor tyrosine kinase, is involved in various signaling pathways that promote tumor cell survival, proliferation, and metastasis. Its expression is commonly found in hematologic malignancies and certain solid tumors but is largely absent in normal adult tissues, making it an ideal target for cancer treatment with minimal off-target effects.
The first key step in Zilovertamab’s mechanism of action is the binding of the monoclonal antibody to ROR1. This binding prevents ROR1 from interacting with its natural ligands, which are critical for activating downstream signaling pathways that facilitate tumor growth and survival. By blocking these interactions, Zilovertamab halts the activation of survival pathways, such as the PI3K/Akt and MAPK/ERK pathways, which are essential for cancer cells to evade apoptosis and continue proliferating.
Second, Zilovertamab induces apoptosis in tumor cells by disrupting the signaling pathways that allow them to resist cell death. This helps to reduce the overall tumor burden and can lead to tumor regression in preclinical models. Furthermore, Zilovertamab has an immune-enhancing effect by marking ROR1-expressing cells, which enhances the recognition and destruction of these tumor cells by the immune system, particularly by cytotoxic T cells. This immune activation adds another layer of therapeutic benefit to the treatment.
Lastly, ongoing research suggests that Zilovertamab may be most effective when used in combination with other therapies, such as Bruton's tyrosine kinase (BTK) inhibitors and immune checkpoint inhibitors. These combinations may synergistically enhance treatment responses, making Zilovertamab a potentially powerful agent in cancer immunotherapy. Overall, its unique mechanism of action offers a promising treatment option for various types of cancers, particularly those that express ROR1.
3.) Clinical Applications of Zilovertamab
Zilovertamab is currently under investigation for its potential clinical applications in a variety of cancers, particularly hematologic malignancies, where it has shown promise in early trials. Its specificity for ROR1-positive cells, which are present in several types of cancer but absent in normal adult tissues, makes it an appealing candidate for targeted therapy. The monoclonal antibody has been studied in combination with other treatment modalities to improve patient outcomes and tackle cancers that have limited treatment options.
In chronic lymphocytic leukemia (CLL), Zilovertamab has demonstrated efficacy in targeting ROR1-expressing tumor cells. When combined with Bruton's tyrosine kinase (BTK) inhibitors like ibrutinib, the therapy enhances the anti-tumor response, offering a new treatment option for patients who may not have responded well to traditional treatments. This combination approach is particularly valuable in CLL, a cancer that often becomes resistant to chemotherapy over time.
Zilovertamab also shows promise in treating mantle cell lymphoma (MCL), another hematologic malignancy. The antibody targets ROR1-positive MCL cells, offering potential benefits for patients who have few available treatment options, especially those who have experienced relapse or refractory disease. The ability to specifically target ROR1-expressing tumor cells provides a new avenue for therapy in this challenging cancer.
Beyond hematologic cancers, Zilovertamab is being explored for its impact on solid tumors, including triple-negative breast cancer (TNBC) and non-small cell lung cancer (NSCLC). Both of these cancers are often difficult to treat and associated with poor prognosis, but early research suggests that Zilovertamab may help address these challenges by targeting ROR1-expressing tumor cells. As clinical trials continue, ongoing research will refine dosing strategies, explore the effectiveness of combination therapies, and expand the potential use of Zilovertamab in the treatment of various cancers. The promising early results signal that Zilovertamab may soon become a valuable addition to the oncology treatment arsenal.
4.) Exploring Biosimilars for Zilovertamab
What is a Biosimilar?
A biosimilar is a highly similar version of an existing biologic drug, designed to match its safety, efficacy, and mechanism of action while offering more accessible research options.
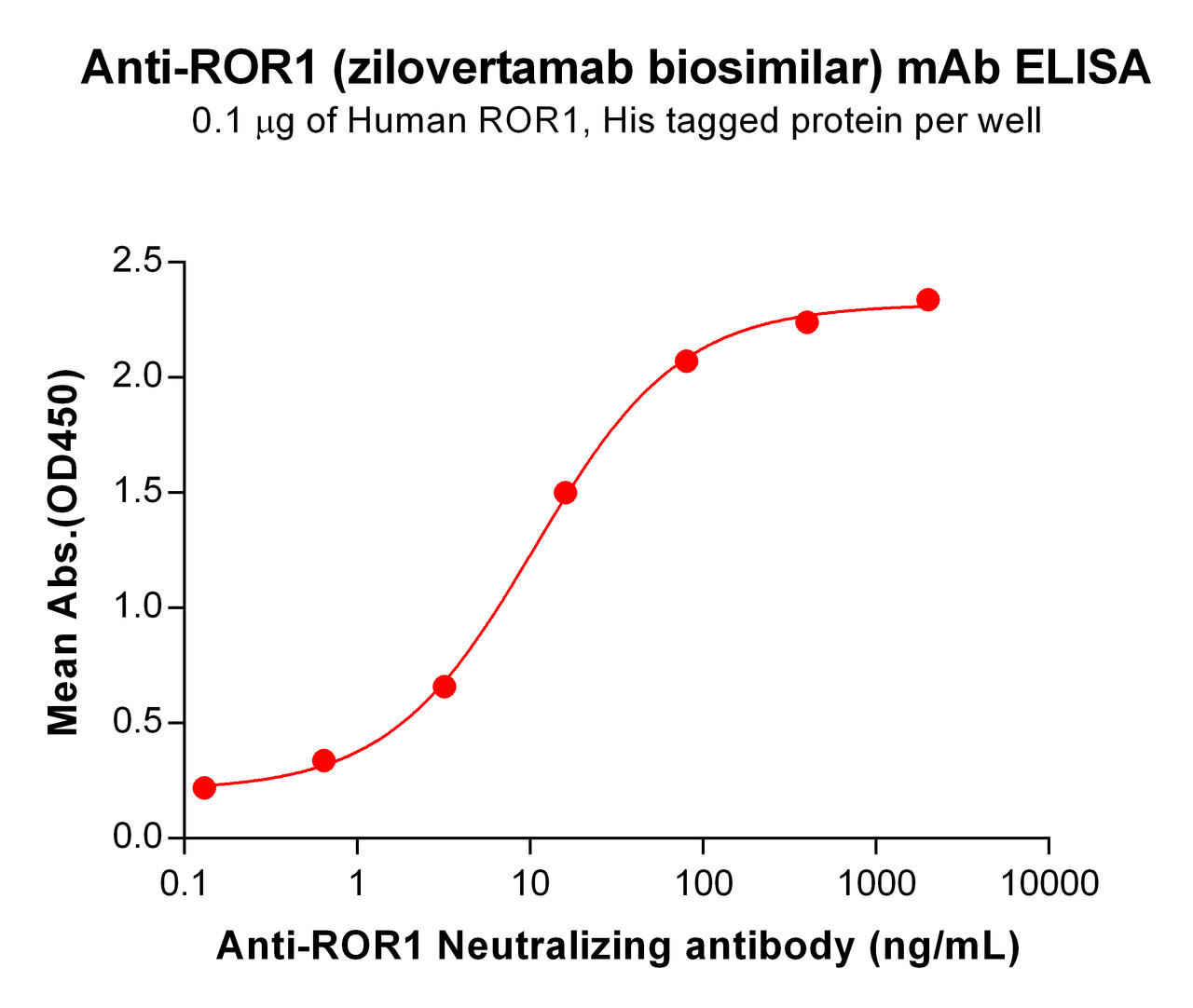
| Zilovertamab (Anti-ROR1) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | ROR1 |
| Reactivity: | Human |
How Zilovertamab Biosimilars Compare
Biosimilars undergo rigorous testing to ensure they mirror the original drug’s pharmacokinetics and biological effects. While not identical, they provide a cost-effective alternative for research and preclinical studies.
Benefits for Research
- Increased Availability: Enhances accessibility for laboratories studying ROR1-targeted therapies.
- Cost-Effective Solutions: Enables broader research applications without the high cost of original biologics.
- Accelerated Discoveries: Supports comparative studies to refine treatment strategies.
Research Use Only Disclaimer:
Zilovertamab biosimilars are intended solely for research purposes and are not approved for clinical use.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Marina Alberto, PhD
Marina Alberto, PhD, holds a robust academic background in Biotechnology, earning her Bachelor’s Degree and PhD in Science and Technology from Quilmes National University. Her research spans cancer immunotherapy, glycan profiling, and vaccine development, including innovative projects on pediatric leukemia diagnosis and cancer-associated carbohydrate-mimetic vaccines. She currently serves as a Technical Support and Sales Specialist at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



