Trastuzumab: Revolutionizing HER2-Positive Cancer Treatment & Research
Quick Facts About Trastuzumab
What is Trastuzumab?
Trastuzumab is a monoclonal antibody used to treat HER2-positive breast and gastric cancers. It specifically targets the HER2 protein, reducing tumor growth and improving survival rates.
How does Trastuzumab work?
Trastuzumab binds to the HER2 receptor on cancer cells, blocking growth signals and triggering immune responses that destroy tumor cells.
What are the clinical applications of Trastuzumab?
It is FDA-approved for HER2-positive breast cancer and metastatic gastric cancer, often used in combination with chemotherapy.
Is Trastuzumab safe?
While generally well-tolerated, it may cause side effects like cardiotoxicity and infusion reactions. Regular monitoring helps manage risks.
1.) Understanding Trastuzumab
Trastuzumab is a breakthrough in oncology, offering targeted therapy for HER2-positive cancers, which tend to be aggressive. By selectively targeting HER2 receptors, Trastuzumab enhances treatment efficacy while reducing the systemic toxicity often seen with traditional chemotherapy. This precision has made it a cornerstone in breast cancer treatment, either alone or in combination with chemotherapy or other HER2-targeted therapies.
Ongoing research continues to explore its potential in different cancer types and optimizing treatment regimens. For example, studies are investigating its use in combination with immune checkpoint inhibitors to enhance therapeutic effects. Trastuzumab has also paved the way for next-generation HER2-targeted therapies, such as Trastuzumab emtansine (T-DM1) and Trastuzumab deruxtecan, which combine the monoclonal antibody with cytotoxic agents for improved efficacy.
In addition to breast cancer, researchers are examining Trastuzumab’s potential in other HER2-expressing tumors, including ovarian, lung, and colorectal cancers. Clinical trials are ongoing to assess the benefits of combination strategies that may overcome resistance mechanisms. For instance, dual HER2 blockade with Pertuzumab and Trastuzumab has demonstrated improved survival rates in certain patient populations. Furthermore, novel delivery methods, such as subcutaneous formulations, aim to enhance patient convenience and adherence. These advancements reinforce Trastuzumab’s critical role in personalized medicine and its potential to shape future oncological treatments. As its clinical applications expand, researchers are also investigating ways to mitigate potential side effects, such as cardiotoxicity, through modified dosing regimens and cardioprotective strategies.
2.) Mechanism of Action of Trastuzumab
Trastuzumab functions by binding to the extracellular domain of the HER2 receptor, preventing ligand-independent signaling and dimerization, which are crucial for tumor cell proliferation. This inhibition halts cancer progression by:
- Blocking HER2 receptor activation, thereby reducing uncontrolled cell division.
- Flagging cancer cells for immune-mediated destruction via antibody-dependent cellular cytotoxicity (ADCC).
- Inducing internalization and degradation of HER2 receptors, leading to decreased oncogenic signaling.
Trastuzumab’s specificity minimizes damage to healthy tissues, distinguishing it from traditional chemotherapies. However, resistance mechanisms, such as HER2 mutations or alternative signaling pathways, remain areas of active research, driving efforts to enhance therapeutic outcomes through combination therapies.
Recent studies suggest that Trastuzumab can modulate the tumor microenvironment, influencing immune responses that support long-term remission. It has also been noted that prolonged exposure to Trastuzumab can result in adaptive resistance, necessitating the integration of additional agents like PI3K inhibitors or anti-PD-1 checkpoint inhibitors to sustain efficacy. Additionally, researchers are investigating antibody-drug conjugates (ADCs) that expand Trastuzumab’s capabilities by delivering potent cytotoxic payloads directly to cancer cells. These evolving insights into its mechanism of action are crucial for refining its clinical applications and overcoming therapeutic limitations.
Moreover, ongoing advancements in biomarker research are helping identify patients who would benefit most from Trastuzumab-based therapies. Understanding the interplay between HER2 signaling and other oncogenic pathways enables precision medicine approaches, improving treatment personalization. This research not only enhances efficacy but also contributes to minimizing toxicity and improving patient outcomes in the long term.
3.) Clinical Applications of Trastuzumab
Trastuzumab is a cornerstone in treating HER2-positive cancers. Clinical applications include:
- Early-Stage Breast Cancer: Used in adjuvant and neoadjuvant settings to reduce recurrence risk. Patients receiving Trastuzumab following surgery and chemotherapy have demonstrated significantly improved disease-free survival rates. Its integration into standard treatment protocols has transformed the prognosis for HER2-positive breast cancer patients, leading to higher long-term survival rates.
- Metastatic Breast Cancer: Combined with chemotherapy for prolonged survival. It is often used with agents like taxanes to enhance its efficacy, and dual HER2 blockade with Pertuzumab has further extended overall survival in advanced cases. The combination of Trastuzumab with newer targeted therapies continues to redefine treatment paradigms, offering hope for better disease management.
- Gastric Cancer: Approved for HER2-positive metastatic gastric and gastroesophageal junction cancers. Studies indicate that Trastuzumab, in combination with platinum-based chemotherapy, enhances survival outcomes in this patient population. Research is ongoing to determine its potential role in earlier-stage gastric cancers and to refine treatment regimens.
Emerging research focuses on optimizing dosing schedules, reducing side effects, and exploring combination therapies with novel agents such as tyrosine kinase inhibitors and immunotherapies. The development of biosimilars further enhances accessibility, ensuring broader patient reach and sustainable treatment options.
Additionally, investigators are exploring alternative dosing regimens to maintain efficacy while minimizing cardiotoxicity, one of Trastuzumab’s known risks. This includes strategies like de-escalation therapy for patients with lower-risk profiles. Personalized medicine approaches, leveraging biomarkers to tailor Trastuzumab therapy, are also under investigation to refine treatment precision. With its expanding applications and continuous innovations, Trastuzumab remains an essential agent in oncology, shaping the landscape of targeted cancer therapy.
As research advances, the integration of artificial intelligence and big data analytics in oncology is further refining Trastuzumab’s clinical applications. Predictive models based on genomic and proteomic data can enhance patient stratification, ensuring that the right treatment is delivered to the right patient at the right time. These developments underscore Trastuzumab’s role not only as a therapeutic but as a driver of innovation in cancer treatment.
4.) Advancing Research on Trastuzumab with Biosimilars
What is a Biosimilar?
Biosimilars are biologic medicines highly similar to an existing approved biologic, offering comparable safety and efficacy. They enhance treatment accessibility while maintaining therapeutic integrity.
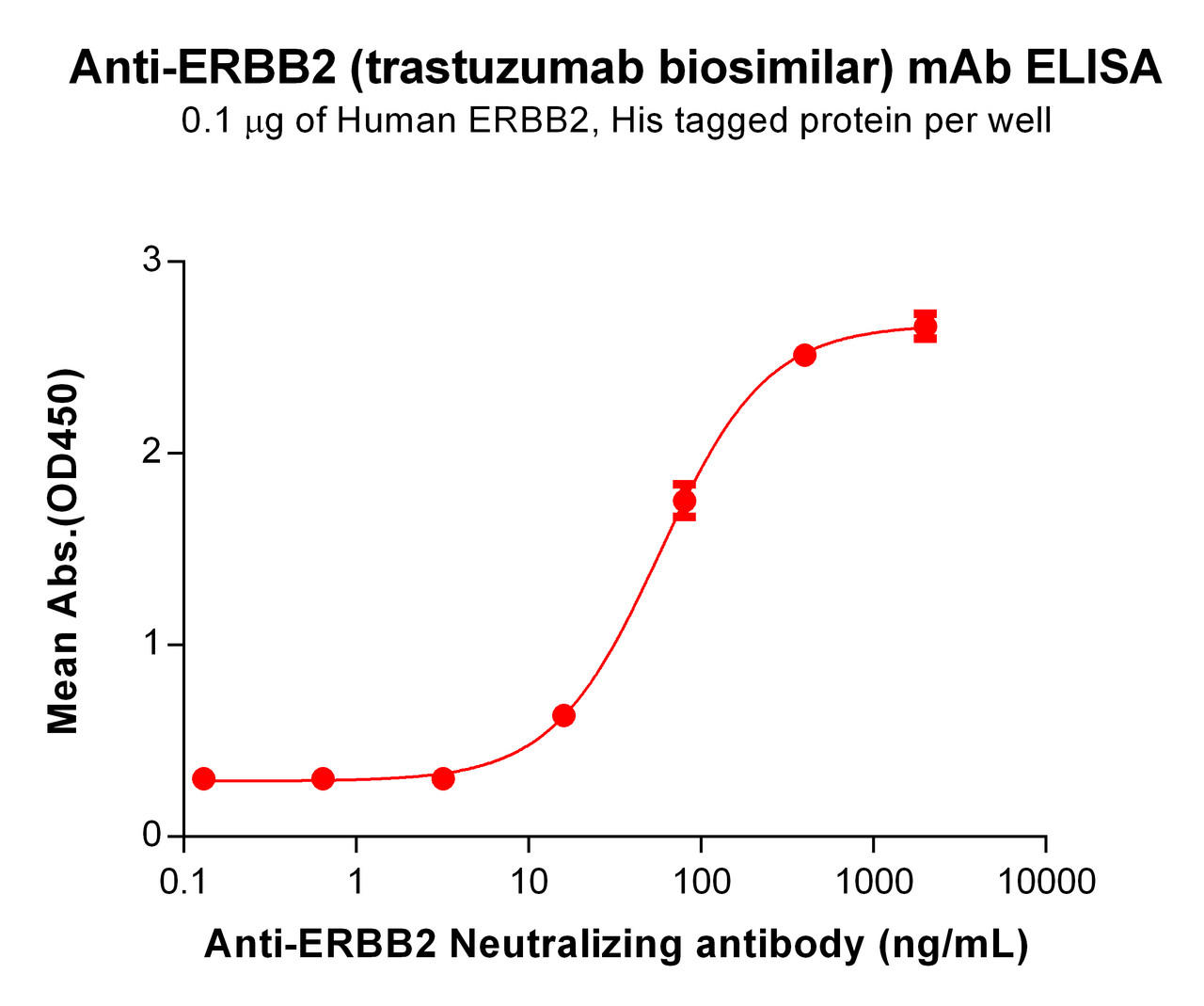
| Trastuzumab (Anti-Her2) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | HER-2 |
| Reactivity: | Human |
How Trastuzumab Biosimilars Compare
Biosimilars undergo rigorous testing to ensure similarity to the reference product. Key benefits include:
- Increased accessibility and affordability.
- Comparable efficacy and safety profiles.
- Expanded research opportunities for novel therapeutic strategies.
Advancing Research on Trastuzumab
Biosimilars provide a crucial tool for research, allowing scientists to:
- Investigate combination therapies and resistance mechanisms.
- Develop predictive biomarkers for personalized treatment.
- Improve manufacturing processes for enhanced stability and delivery.
Research Use Only Disclaimer:
Trastuzumab biosimilars for research purposes should be used strictly within regulatory guidelines and intended applications.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Miren Ruiz de Eguilaz, PhD
Miren Ruiz de Eguilaz, PhD, has an extensive academic background, earning a BSc in Biology from UPV/EHU, an MSc in Biotechnology from the University of Oviedo, and a PhD in Chemistry from Dublin City University (DCU). Miren’s expertise lies in biosensor technology and bacterial diagnostics. She currently serves as a Product Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



