TNFRSF9: Enhancing Immune Cell Activity Against Tumors
Introduction to TNFRSF9 and Its Role in Immune Activation
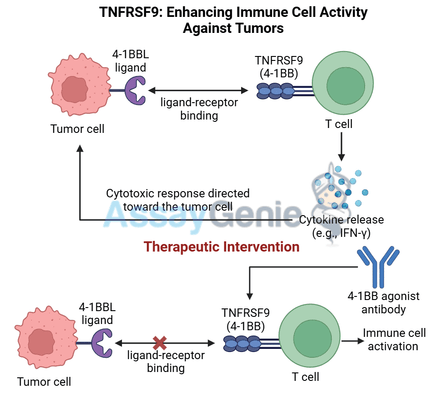
TNFRSF9, also known as 4-1BB or CD137, is a co-stimulatory receptor expressed on the surface of T cells, natural killer (NK) cells, and dendritic cells. It belongs to the tumor necrosis factor receptor superfamily (TNFRSF), which plays critical roles in regulating immune responses. Activation of TNFRSF9 boosts the activity and survival of T cells and NK cells, making it a powerful target in cancer immunotherapy where enhanced anti-tumor immunity is essential for effective treatment.
When TNFRSF9 binds to its ligand, 4-1BBL, expressed on antigen-presenting cells (APCs), it delivers a robust co-stimulatory signal that promotes T cell expansion, cytokine production, and cytotoxic activity. These immune-enhancing properties have made TNFRSF9 an attractive target for monoclonal antibodies, such as B3113, that activate TNFRSF9 signaling to amplify anti-tumor responses.
This article explores the biology of TNFRSF9, its functions in T cell and NK cell activation, and the potential of TNFRSF9-targeting therapies like B3113 in cancer treatment.
TNFRSF9: Structure and Function in Immune Activationh
The Role of TNFRSF9 in T Cell Activation
TNFRSF9 is primarily expressed on activated T cells and NK cells, where it plays an essential role in boosting immune cell responses. Following initial T cell activation through the T cell receptor (TCR), TNFRSF9 engagement provides a second co-stimulatory signal that enhances T cell proliferation, survival, and effector function. Key effects of TNFRSF9 activation include:
- Enhanced T cell proliferation: TNFRSF9 signaling supports the expansion of T cells, particularly CD8+ cytotoxic T cells that target and kill tumor cells.
- Increased cytokine production: Activation of TNFRSF9 promotes the secretion of pro-inflammatory cytokines like IFN-γ and TNF-α, which amplify the immune response and directly inhibit tumor growth.
- Extended T cell survival: TNFRSF9 engagement prevents T cell apoptosis, allowing T cells to persist longer within the tumor microenvironment and sustain their anti-tumor activity.
This robust co-stimulatory signal provided by TNFRSF9 is crucial for the generation of effective anti-tumor immunity, especially in cancers where immune cells are often suppressed by the tumor microenvironment.
The Role of TNFRSF9 in NK Cell Activation
In addition to its effects on T cells, TNFRSF9 also enhances the activity of natural killer (NK) cells, which play a vital role in innate immune responses against tumors. TNFRSF9 signaling in NK cells leads to:
- Increased cytotoxicity: TNFRSF9 activation boosts the cytotoxic potential of NK cells, enhancing their ability to recognize and destroy cancer cells.
- Improved cytokine secretion: Like T cells, NK cells activated through TNFRSF9 produce higher levels of IFN-γ, which promotes anti-tumor immunity and supports T cell function.
- Enhanced survival: TNFRSF9 signaling supports the survival and persistence of NK cells, allowing them to continuously patrol and attack tumor cells.
Through its effects on both T cells and NK cells, TNFRSF9 is a critical checkpoint in the immune response to cancer, capable of amplifying the body’s natural defense mechanisms against tumors.
TNFRSF9 in the Tumor Microenvironment
TNFRSF9 and Immune Suppression in Cancer
In the tumor microenvironment (TME), cancer cells and tumor-associated immune cells often exploit immune checkpoints to suppress T cell and NK cell function. Although TNFRSF9 signaling can boost immune activation, its expression is tightly regulated and often only occurs upon T cell and NK cell activation. Tumors may produce immunosuppressive cytokines and recruit regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), which inhibit TNFRSF9 signaling pathways, dampening the immune response.
The immunosuppressive nature of the TME, combined with the limited and regulated expression of TNFRSF9, underscores the need for therapeutic interventions that can selectively activate TNFRSF9 to overcome immune evasion.
Therapeutic Potential of Targeting TNFRSF9 in Cancer
Therapies targeting TNFRSF9 aim to activate T cells and NK cells within the TME, enhancing their anti-tumor activity despite the immunosuppressive signals present. By engaging TNFRSF9 with agonistic antibodies like B3113, these therapies can deliver a targeted co-stimulatory signal to boost immune cell function without the systemic effects seen with broader immune stimulants.
Potential benefits of TNFRSF9-targeting therapies include:
- Reinvigorating T cell responses: Targeting TNFRSF9 enhances the expansion and survival of T cells, particularly cytotoxic CD8+ T cells, which are essential for tumor clearance.
- Activating NK cells: TNFRSF9 signaling in NK cells boosts their cytotoxic function, increasing
their ability to attack and kill tumor cells directly.
B3113: A Monoclonal Antibody Targeting TNFRSF9
Mechanism of Action of B3113
https://www.assaygenie.com/human-tnf-alpha-elisa-kit/B3113 is an agonistic monoclonal antibody that specifically binds to TNFRSF9, mimicking the natural ligand (4-1BBL) and activating the receptor. The mechanism of action of B3113 involves:
- Direct activation of TNFRSF9: B3113 binds to TNFRSF9 on T cells and NK cells, delivering a co-stimulatory signal that enhances their proliferation, survival, and cytotoxic function.
- Boosting cytokine production: By activating TNFRSF9, B3113 promotes the release of key cytokines such as IFN-γ and TNF-α, which support immune cell recruitment and function within the TME.
- Enhancing T cell and NK cell cytotoxicity: B3113-activated immune cells show increased cytotoxicity against tumor cells, directly improving anti-tumor responses.
Clinical Applications of B3113
The therapeutic potential of B3113 is being explored in cancers with high levels of immune suppression in the TME, such as:
- Melanoma: Known for its immunosuppressive environment, melanoma can be effectively
targeted by TNFRSF9 agonists to boost T cell and NK cell responses. - Non-Small Cell Lung Cancer (NSCLC): High levels of Tregs and MDSCs in NSCLC can inhibit
immune responses; targeting TNFRSF9 with B3113 may enhance immune cell activation and improve treatment outcomes. - Hepatocellular Carcinoma (HCC): In HCC, TNFRSF9-targeting therapies can activate NK cells and T cells to counteract liver-specific immune suppression and attack tumor cells.
Cancer Type | TNFRSF9 Expression | Therapeutic Potential of TNFRSF9 Agonist (B3113) |
|---|---|---|
Expressed on T cells and NK cells in TME | B3113 enhances T cell and NK cell function to overcome immune suppression. | |
Suppressed in high Treg and MDSC environments | TNFRSF9 activation boosts T cell responses and enhances anti-tumor activity. | |
Immune suppression in liver-specific TME | B3113 increases NK cell and T cell cytotoxicity in liver cancer. |
Synergy with Checkpoint Inhibitors and Other Therapies
Combination with Checkpoint Inhibitors
Checkpoint inhibitors like anti-PD-1 and anti-CTLA-4 are widely used in cancer treatment to remove inhibitory signals that limit T cell activation. Targeting TNFRSF9 with B3113 complements checkpoint inhibitors by directly enhancing T cell and NK cell activity, which can be especially beneficial in tumors resistant to checkpoint blockade alone.
- Enhanced T cell activation: B3113 boosts T cell activity and survival, making these cells more responsive to checkpoint inhibition.
- Overcoming resistance: In tumors where checkpoint inhibitors alone are insufficient, TNFRSF9 activation provides an additional layer of immune enhancement, helping to overcome immune evasion mechanisms in the TME.
Potential for Combination with CAR-T Cell Therapy
Chimeric Antigen Receptor T cell (CAR-T) therapy involves engineering T cells to express specific receptors that target tumor cells. However, CAR-T cell efficacy is often limited in solid tumors due to the immunosuppressive TME. Targeting TNFRSF9 with B3113 can enhance CAR-T cell function by increasing their survival and activity within the TME, potentially improving outcomes in CAR-T cell-treated patients.
Challenges and Future Directions in TNFRSF9-Targeted Therapy
Managing Immune-Related Toxicities
As with other immune-stimulating therapies, activating TNFRSF9 with B3113 could lead to immune-related adverse events (irAEs), such as excessive inflammation or cytokine release syndrome (CRS). Careful patient monitoring, dose optimization, and selective activation of TNFRSF9 will be critical to maximize therapeutic efficacy while minimizing risks.
Expanding Applications in Other Cancer Types
Future studies will likely explore the broader application of TNFRSF9-targeting therapies in other cancers with high levels of immune suppression. Biomarker studies to identify patients most likely to benefit from TNFRSF9 agonists will also be essential, allowing for more personalized approaches in cancer immunotherapy.
Conclusion
TNFRSF9 (4-1BB) is a powerful immune co-stimulatory receptor that enhances T cell and NK cell function, making it an important target in cancer immunotherapy. By targeting TNFRSF9 with therapies like B3113, researchers aim to amplify immune cell responses, overcome tumor-induced immune suppression, and enhance the effectiveness of existing cancer treatments, such as checkpoint inhibitors and CAR-T therapies. As research advances, TNFRSF9-targeted therapies hold great potential for expanding the cancer immunotherapy landscape and improving outcomes for patients with immune-resistant tumors.
References
- Chester, C., et al., 2018. 4-1BB agonists in cancer immunotherapy. Journal for Immunotherapy of Cancer, 6(1), pp.39-52.
- Vinay, D.S., Kwon, B.S., 2014. Therapeutic potential of 4-1BB (CD137) in cancer. Seminars in Oncology, 41(5), pp.532-538.
- Liu, L., et al., 2016. TNFRSF9 signaling: A key pathway in cancer immunotherapy. Trends in Immunology, 37(6), pp. 407-417.
- Melero, I., et al., 2015. Therapeutic strategies to enhance anti-tumor immunity by targeting CD137 (4-1BB) signaling. Cytokine & Growth Factor Reviews, 26(2), pp.137-145.
- Guo, Z., et al., 2014. Targeting CD137 for T cell cancer immunotherapy. Journal of Hematology & Oncology, 7(1), pp.18-26.
- Sharma, P., Allison, J.P., 2015. The future of immune checkpoint therapy. Science, 348(6230), pp.56-61.
- Choi, B.K., Lee, D.Y., Lee, D.G., et al., 2011. 4-1BB-based immunotherapy in solid tumors. Current Opinion in Immunology, 23(2), pp.203-209.
- Pardoll, D.M., 2012. The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer, 12(4), pp.252-264.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Praluzatamab: Unveiling the Promise of CD47-Targeted Therapy in Cancer Research
Quick Facts About PraluzatamabWhat is Praluzatamab?Praluzatamab is an experimental mon …13th May 2025