Tinurilimab: Advancing Immunotherapy Through CD47 Blockade
Quick Facts About Tinurilimab
What is Tinurilimab?
Tinurilimab is a monoclonal antibody targeting CD47, a protein that cancer cells use to evade immune system attack. By blocking CD47, Tinurilimab enhances the immune response against tumors.
How does Tinurilimab work?
It inhibits CD47’s interaction with SIRPα, allowing macrophages to recognize and destroy cancer cells more effectively.
What are the clinical applications of Tinurilimab?
Is Tinurilimab safe?
Ongoing clinical trials are assessing its safety and efficacy. Early results suggest it has a manageable safety profile, though risks like anemia require monitoring.
1.) Understanding Tinurilimab
Tinurilimab is an innovative immunotherapeutic agent that belongs to a new class of treatments targeting CD47, a key protein that plays a central role in immune evasion. Often referred to as the “don’t eat me” signal, CD47 is frequently overexpressed on tumor cells, effectively preventing the immune system from recognizing and attacking these malignant cells. This overexpression of CD47 helps cancer cells evade destruction by signaling immune cells, like macrophages, to ignore them. By blocking the interaction between CD47 and its receptor, SIRPα, Tinurilimab counteracts this immune evasion mechanism, allowing macrophages and other immune cells to effectively target and clear tumor cells.
The potential of Tinurilimab as a therapeutic agent in oncology lies in its ability to restore the body’s natural immune response to cancer. Tumors that have become resistant to conventional therapies, including chemotherapy and certain immunotherapies, often exploit CD47 as a means of immune suppression. By inhibiting CD47, Tinurilimab provides a novel method of overcoming this resistance, reactivating the immune system’s innate ability to recognize and eliminate cancerous cells. Additionally, research suggests that Tinurilimab may work most effectively in combination with other therapies, such as PD-1 inhibitors and chemotherapeutic agents. These combination therapies could provide a synergistic effect, potentially offering more comprehensive treatment strategies for patients with relapsed or difficult-to-treat cancers. As clinical research into CD47 blockade progresses, Tinurilimab holds promise as a valuable tool in the arsenal of cancer immunotherapy, particularly for patients with cancers that have not responded to traditional treatments.
2.) Mechanism of Action of Tinurilimab
The mechanism of action of Tinurilimab is based on its ability to block the interaction between CD47, a “don’t eat me” signal, and its receptor, SIRPα, found on macrophages. Under normal circumstances, CD47 helps protect healthy cells from being targeted by the immune system by signaling immune cells to avoid phagocytosis. However, in the context of cancer, this mechanism allows tumor cells to evade immune surveillance and continue to grow unchecked. By inhibiting the CD47-SIRPα interaction, Tinurilimab effectively removes this immune checkpoint, enabling macrophages and other immune cells to detect and attack cancer cells more efficiently.
This blockade of CD47 is significant because it enhances innate immunity, which contrasts with the approach of many traditional cancer therapies that focus on T-cell activation. While immune checkpoint inhibitors, like PD-1 blockers, work by reactivating T-cells, Tinurilimab operates at the level of the innate immune system, particularly macrophages. This distinction makes Tinurilimab a promising option for patients who do not respond to T-cell-based therapies. Macrophages, which play a vital role in the body’s first line of defense, are able to recognize and engulf cancer cells once CD47 inhibition is achieved. This mechanism not only enhances the immune response but also offers a potentially more effective treatment option for a broader range of cancers, particularly those resistant to conventional treatments. In combination with other therapies, Tinurilimab’s ability to amplify innate immune responses may provide significant therapeutic benefits for cancer patients.
3.) Clinical Applications of Tinurilimab
Tinurilimab is being investigated in clinical trials across various types of cancers, including both hematologic malignancies and solid tumors. One of its most promising applications is in the treatment of hematologic malignancies, such as leukemia and lymphoma, where early-phase trials have shown positive results in patients with relapsed or refractory disease. These cancers often present challenges in treatment due to their ability to evade immune responses, making CD47 blockade an attractive therapeutic strategy. Tinurilimab's ability to enhance macrophage-mediated phagocytosis may help overcome this immune resistance and improve outcomes for patients with these challenging blood cancers.
In addition to hematologic malignancies, Tinurilimab is being evaluated for its potential in treating solid tumors. Research is currently exploring its use in lung cancer, breast cancer, and colorectal cancer, among others. In these cancers, CD47 is frequently overexpressed, contributing to tumor growth and immune escape. By inhibiting CD47, Tinurilimab could improve immune infiltration into tumors, enhance the effectiveness of existing therapies, and potentially overcome resistance to checkpoint inhibitors, such as PD-1/PD-L1 inhibitors. Preclinical data suggest that Tinurilimab may work synergistically with other immunotherapies, improving patient outcomes in combination treatments.
As clinical trials progress, optimizing dosing strategies will be essential to maximize the therapeutic potential of Tinurilimab. One challenge for patients receiving CD47-targeting therapies is the potential for side effects, such as anemia, which occurs due to the role of CD47 in red blood cell survival. Researchers are actively working to refine dosing protocols to mitigate these side effects while maintaining the drug’s efficacy in combating cancer. With ongoing studies and further exploration of its clinical applications, Tinurilimab holds great promise as a key player in the fight against a wide variety of cancers.
4.) Exploring Biosimilars for Tinurilimab
What is a Biosimilar?
A biosimilar is a biologic product highly similar to an existing approved reference biologic, with no clinically meaningful differences in safety or effectiveness. Biosimilars provide a cost-effective alternative for research and therapeutic development.
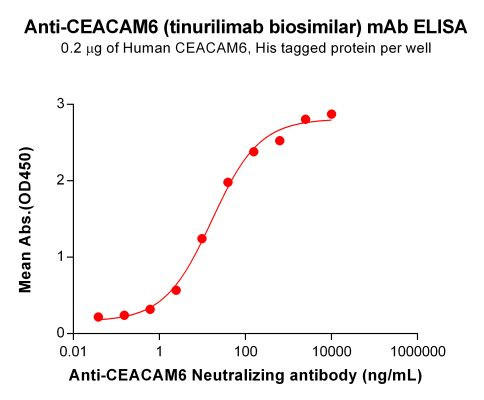
| Tinurilimab (Anti-CEACAM6) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | CEACAM6 |
| Reactivity: | Human |
How Tinurilimab Biosimilar Compares to Tinurilimab
Tinurilimab biosimilar is designed to closely replicate the original molecule’s structure and function, offering researchers a valuable tool for studying CD47 inhibition. While it is not approved for clinical use, it serves as an essential asset in preclinical and translational research.
Benefits of Tinurilimab Biosimilar for Research
- Cost-effective alternative for laboratory studies
- Reliable consistency for testing CD47 blockade mechanisms
- Supports innovation in combination therapy development
Advancing Research on Tinurilimab
By providing researchers with a high-quality alternative to the reference product, Tinurilimab biosimilar facilitates new discoveries in immuno-oncology. It enables a deeper understanding of CD47 blockade, aiding the development of next-generation therapies.
Research Use Only Disclaimer:
Tinurilimab biosimilar is not intended for human therapeutic use.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Chris McNally, PhD
Chris McNally, PhD, has a strong foundation in Biomedical Science, completing a PhD scholarship in collaboration with Randox Laboratories and Ulster University. Chris has published extensively in prostate cancer research, focusing on biomarker discovery, cancer risk stratification, and molecular mechanisms such as hypoxia-induced regulation. He currently serves as a Business Development Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



