The PD-1 Pathway and Cancer Immunotherapy
The initial identification of PD-1 as a potential target for cancer treatment occurred in the late 1990s and early 2000s through studies exploring the regulation of T-cell responses. The development and approval of PD-1 inhibitors, such as pembrolizumab and nivolumab, for the treatment of various cancers have since transformed the landscape of cancer immunotherapy. The use of PD-1 pathway inhibitors in cancer treatment has rapidly gained recognition and has become a cornerstone of modern immunotherapeutic approaches.
Table of Contents
Jump to a section:
What is the PD-1 Pathway?
PD-1 (Programmed Cell Death Protein 1), is a cell surface receptor primarily found on T cells, a type of white blood cell responsible for orchestrating the immune response. When activated, PD-1 acts as an immune checkpoint, regulating the intensity and duration of immune responses. It serves as a key mechanism to prevent excessive immune activation and maintain immune homeostasis. The PD-1 pathway is a crucial element of the immune system's regulation and response to various diseases, including cancer.
PD-L1 and PD-L2
The PD-1 pathway involves interactions between PD-1, a cell surface receptor, and its ligands, PD-L1 (Programmed Death-Ligand 1) and PD-L2 (Programmed Death-Ligand 2). PD-L1 and PD-L2 are proteins expressed on the surface of various cells, including immune cells and cancer cells. When PD-1 interacts with its ligands, PD-L1 and PD-L2, which are expressed on the surface of various cells, it triggers an inhibitory signal that dampens the immune response. PD-L1 and PD-L2 act as checkpoints by binding to PD-1, thereby preventing T cells from launching an overly aggressive immune attack. This signaling plays a crucial role in maintaining immune tolerance and preventing unwarranted immune activation against healthy tissues.
The PD-1 Pathway in Cancer
The PD-1 pathway assumes a critical role in tumor immune evasion and contributes to the challenges in effectively combating cancer. Cancer cells have developed sophisticated strategies to exploit this pathway as a means of evading immune surveillance and resisting destruction by the immune system. One of the key mechanisms employed by cancer cells is the upregulation of PD-L1 expression on their surface. This upregulation allows cancer cells to engage with PD-1 on T cells, triggering an inhibitory signal that suppresses the activity of the T cells.
The interaction between PD-L1 on cancer cells and PD-1 on T cells induces a signaling cascade that disables the immune system's ability to recognize and eliminate cancer cells. By suppressing the activity of T cells, which play a crucial role in orchestrating the immune response against tumors, cancer cells can evade immune detection and destruction. This process hampers the body's natural defense mechanisms, allowing tumors to grow and proliferate without encountering robust immune responses.
Additionally, the inhibitory signals triggered by the PD-1 pathway dampen the effectiveness of anti-tumor immune responses. This prevents T cells from mounting an effective anti-tumor immune response, which would typically involve recognizing and eliminating cancer cells. As a result, the immune system becomes less capable of controlling the growth and spread of cancer cells, contributing to disease progression.
Immune Checkpoints
Immune checkpoints are molecules or receptors expressed on immune cells that regulate the intensity and duration of immune responses. Immune checkpoints act as molecular brakes, balancing the immune system's activation and inhibition signals to ensure an appropriate immune response. PD-1 is an immune checkpoint receptor that regulates immune responses by interacting with its ligands, PD-L1 and PD-L2. The PD-1/PD-L1 interaction is a crucial checkpoint mechanism that prevents excessive immune activation and maintains immune homeostasis. Beyond the PD-1/PD-L1 pathway, other important immune checkpoints, such as CTLA-4 (Cytotoxic T Lymphocyte Antigen 4), LAG-3 (Lymphocyte-activation gene 3), TIM-3 (T-cell Immunoglobulin and Mucin-domain containing-3), and others, have garnered significant attention in the field of cancer immunotherapy. These checkpoints serve as molecular brakes that can dampen or suppress immune responses to prevent excessive activation and immune-mediated damage. However, tumors often exploit these checkpoints to evade immune surveillance and establish an immunosuppressive microenvironment. By targeting these additional immune checkpoints, novel therapeutic strategies aim to enhance anti-tumor immune responses and overcome resistance mechanisms encountered with single checkpoint blockade.
To harness the potential of immune checkpoints for cancer treatment, monoclonal antibodies have been developed to block the activity of these checkpoint proteins. By targeting immune checkpoint proteins, including PD-1, PD-L1, and CTLA-4, with monoclonal antibodies, the immune system can overcome cancer's ability to resist immune responses. This blockade restores the immune system's ability to recognize and attack cancer cells, enabling the body's own defense mechanisms to remain effective in its defenses against cancer.
Immune Checkpoint
PD-1 and PD-L1 in Cancer Immunotherapy
Immunotherapy is a medical approach that harnesses the power of the immune system to fight diseases, particularly cancer. Researchers have developed innovative therapies known as PD-1 inhibitors or PD-1 checkpoint inhibitors, which have revolutionized the landscape of cancer immunotherapy. Additionally,emerging immunotherapy approaches utilize PD-L1 as a target, including combination immunotherapy and adoptive T cell therapy. PD-1 inhibitors specifically target the PD-1 receptor on T cells and block its interaction with PD-L1 or PD-L2 on target cells while PD-L1 inhibitors directly target the PD-L1 molecule expressed on cancer cells.
PD-1 Inhibitors
PD-1 inhibitors, including monoclonal antibodies such as pembrolizumab and nivolumab, have emerged as a revolutionary class of immunotherapies in cancer treatment. These inhibitors work by blocking the interaction between PD-1 and its ligands, such as PD-L1 and PD-L2, thereby preventing the inhibitory signals that suppress the immune response. By releasing the brakes on the immune system, PD-1 inhibitors enable T cells to mount a potent and targeted attack against cancer cells. Notably, PD-1 inhibitors have shown remarkable efficacy in various types of cancer, including melanoma, lung cancer, and kidney cancer, among others. They have demonstrated durable responses, improved survival rates, and even led to long-term remission in some patients. PD-1 inhibitors have also been investigated in combination with other immunotherapies, such as immune checkpoint inhibitors targeting different pathways, as well as with targeted therapies and conventional treatments like chemotherapy. These combinatorial approaches aim to enhance treatment responses and overcome resistance mechanisms employed by cancer cells, offering new possibilities in the fight against cancer.
Approved PD-1 inhibitors:
PD-L1 Inhibitors
PD-L1 inhibitors, such as atezolizumab and pembrolizumab, are monoclonal antibody therapies that work by blocking PD-L1 on cancer cells, disrupting its binding with PD-1 on T cells, and reinvigorating the anti-tumor immune response. By blocking this inhibitory pathway, PD-L1 therapies enhance the immune system's ability to recognize and attack cancer cells. PD-L1 inhibitors have demonstrated significant efficacy in various cancers, including bladder cancer, non-small cell lung cancer, and head and neck cancer. These monoclonal antibody therapies have been shown to improve response rates and overall survival in patients.
Approved PD-L1 inhibitors:
Combination Immunotherapy
Combination immunotherapy approaches, such as combining PD-1 or PD-L1 inhibitors with other immune checkpoint inhibitors like CTLA-4 inhibitors (e.g., ipilimumab), aim to further enhance treatment outcomes. The synergistic effects of these combinations can lead to improved response rates and long-term survival in patients. Additionally, the combination of PD-1 or PD-L1 inhibitors with targeted therapies or conventional treatments like chemotherapy has also shown promising results. These combination strategies work by targeting different aspects of cancer growth and immune regulation, maximizing the chances of a successful response and overcoming resistance mechanisms.
Approved Combination Therapies:
- Pembrolizumab (Keytruda) + Ipilimumab (Yervoy)
- Pembrolizumab (Keytruda) + Axitinib (Inlyta)
- Nivolumab (Opdivo) + Ipilimumab (Yervoy)
Adoptive T-cell Immunotherapy
Adoptive T-cell therapy (ACT) is an innovative approach in cancer treatment that involves genetically modifying patient-derived T cells to express chimeric antigen receptors (CAR-T cells) or T-cell receptors (TCR-T cells) targeting specific tumor antigens. This personalized therapy has shown remarkable success in hematological malignancies, such as leukemia and lymphoma. When combined with PD-1 or PD-L1 inhibitors, adoptive T-cell therapy can further enhance the immune response against cancer cells. The PD-1 or PD-L1 inhibition helps overcome the immunosuppressive tumor microenvironment and sustains the activity of the adoptively transferred T cells, leading to improved therapeutic outcomes.
Approved adoptive T-cell Therapies:
Other Therapeutic Approaches
In addition to the above approaches, there are other emerging strategies being explored in the field of PD-1/PD-L1 immunotherapy. These include cancer vaccines, which aim to stimulate the immune system's recognition of cancer cells and enhance the anti-tumor response. Oncolytic virus therapy involves using genetically engineered viruses to selectively infect and destroy cancer cells, while simultaneously activating the immune system against the tumor. Bispecific antibodies, which can simultaneously bind to PD-1 or PD-L1 and other targets, offer a novel approach to modulating immune responses against cancer. Furthermore, the development of small molecule inhibitors targeting the PD-1/PD-L1 pathway is an area of active research, providing additional therapeutic options for cancer patients.
PD-1 Immunotherapy
PD-1 Related Products
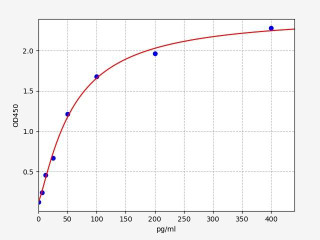
| Human PD-L1 / CD274 ELISA Kit | |
|---|---|
| ELISA TYPE | Sandwich ELISA |
| SENSITIVITY | 3.75pg/ml |
| RANGE | 6.25-400pg/ml |
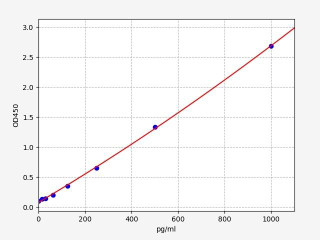
| Human IL-2 ELISA Kit | |
|---|---|
| ELISA TYPE | Sandwich ELISA |
| SENSITIVITY | 9.375pg/ml |
| RANGE | 15.625-1000pg/ml |
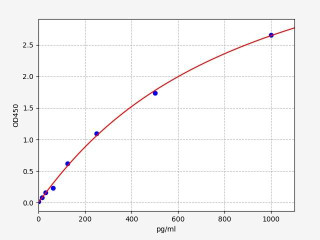
| Human TNF alpha ELISA Kit | |
|---|---|
| ELISA TYPE | Sandwich |
| SENSITIVITY | 9.375pg/ml15.625-1000pg/ml |
| RANGE | 15.625-1000pg/ml |
Written by Lauryn McLoughlin
Lauryn McLoughlin completed her undergraduate degree in Neuroscience before completing her masters in Biotechnology at University College Dublin.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025




