Tezepelumab: A Breakthrough in Severe Asthma Treatment and Research
Quick Facts About Tezepelumab
What is Tezepelumab?
How does Tezepelumab work?
What are the clinical applications of Tezepelumab?
1.) Understanding Tezepelumab
Tezepelumab represents a paradigm shift in severe asthma management. Unlike traditional biologics that target specific inflammatory markers like eosinophils or IgE, Tezepelumab takes a broader approach by inhibiting thymic stromal lymphopoietin (TSLP), a cytokine that activates multiple inflammatory pathways. This mechanism makes it a versatile treatment option for patients who do not respond to existing biologics, offering a more comprehensive approach to inflammation control.
Developed through a collaboration between Amgen and AstraZeneca, Tezepelumab has demonstrated significant clinical benefits, including a substantial reduction in exacerbation rates and improved lung function. Clinical trials such as the PATHWAY and NAVIGATOR studies have confirmed its efficacy across diverse patient populations, including those with low eosinophil counts who typically have limited treatment options. Its broad mechanism of action sets it apart from other biologics, making it one of the most promising therapies for severe asthma.
Tezepelumab is administered via subcutaneous injection, typically once every four weeks, which enhances patient compliance compared to more frequent dosing regimens. Clinical studies indicate that it has been well-tolerated, with a safety profile comparable to placebo. The most common adverse events reported include injection site reactions, nasopharyngitis, and headache, though serious side effects are rare.
Beyond asthma, researchers are actively exploring Tezepelumab’s potential in treating other inflammatory diseases such as eosinophilic esophagitis (EoE) and chronic spontaneous urticaria (CSU). By modulating inflammation at an upstream level, Tezepelumab offers potential benefits for a broad range of conditions driven by epithelial dysfunction and immune system overactivation. Ongoing clinical trials continue to investigate its effectiveness in these and other conditions, expanding the therapeutic potential of this groundbreaking biologic.
2.) Mechanism of Action of Tezepelumab
Tezepelumab exerts its therapeutic effects by targeting TSLP, a cytokine released by epithelial cells in response to allergens, pollutants, and respiratory infections. TSLP plays a crucial role in initiating and sustaining airway inflammation by activating dendritic cells, mast cells, basophils, and other immune components. This cytokine serves as an upstream regulator of type 2 inflammation, which is implicated in the pathogenesis of severe asthma and other inflammatory conditions.
By inhibiting TSLP, Tezepelumab disrupts multiple inflammatory pathways, leading to:
- Reduced eosinophilic and non-eosinophilic inflammation: Unlike other biologics that specifically target eosinophils, Tezepelumab provides broader efficacy by acting earlier in the inflammatory cascade.
- Decreased airway hyperresponsiveness: By reducing inflammation at its source, Tezepelumab helps improve lung function and decreases the frequency of exacerbations.
- Improved patient outcomes across biomarker levels: Unlike IL-5 inhibitors, which primarily benefit patients with high eosinophil counts, Tezepelumab has demonstrated effectiveness even in patients with low eosinophil counts, expanding treatment options for a broader asthma population.
Preclinical studies and large-scale clinical trials have provided compelling evidence of Tezepelumab’s efficacy. The NAVIGATOR study, a pivotal phase 3 trial, showed that Tezepelumab significantly reduced asthma exacerbations by up to 70% compared to placebo, regardless of baseline eosinophil levels. These findings underscore the drug’s potential to transform asthma treatment by addressing inflammation more comprehensively than existing biologics.
3.) Clinical Applications of Tezepelumab
Tezepelumab is primarily approved for the treatment of severe asthma in patients aged 12 years and older. Its unique mechanism makes it an attractive option for individuals who do not respond adequately to traditional biologics targeting IL-5, IL-4, or IgE. By blocking TSLP, Tezepelumab targets inflammation at an upstream level, making it effective across a broader range of asthma phenotypes.
- Reduction in exacerbations: Clinical trials have shown that Tezepelumab can reduce severe asthma attacks by up to 70%, significantly improving patient outcomes.
- Improved lung function: Patients experience enhanced airflow and reduced respiratory symptoms, leading to a better quality of life.
- Broad efficacy across patient populations: Unlike eosinophil-targeting biologics, Tezepelumab is effective in both eosinophilic and non-eosinophilic asthma, making it a viable option for more patients.
Beyond asthma, Tezepelumab is being actively investigated for its potential in treating various inflammatory diseases. Its ability to modulate type 2 inflammation upstream makes it a promising candidate for conditions that share similar immune dysregulation mechanisms.
- Atopic Dermatitis: TSLP plays a key role in skin inflammation, and preliminary research suggests that Tezepelumab could help reduce symptoms in moderate-to-severe atopic dermatitis patients.
- Chronic Rhinosinusitis with Nasal Polyps (CRSwNP): Studies are exploring whether Tezepelumab can reduce nasal inflammation and polyp size in patients suffering from CRSwNP, a condition often linked to type 2 inflammation.
- Eosinophilic Esophagitis (EoE): By reducing esophageal inflammation, Tezepelumab could improve swallowing function and reduce disease severity in patients with EoE.
- Chronic Spontaneous Urticaria (CSU): Researchers are investigating its ability to modulate immune responses that contribute to CSU, a debilitating condition characterized by persistent hives and swelling.
Several ongoing clinical trials continue to assess Tezepelumab’s role in these and other inflammatory conditions, reinforcing its potential as a game-changing biologic therapy. As research progresses, its applications may expand beyond respiratory diseases, offering hope to patients with chronic inflammatory disorders driven by epithelial dysfunction.
4.) Exploring Biosimilars for Tezepelumab
What is a Biosimilar?
A biosimilar is a highly similar version of an existing biologic drug, with no clinically meaningful differences in safety, purity, or potency. Unlike generics, biosimilars require extensive testing to demonstrate equivalence.
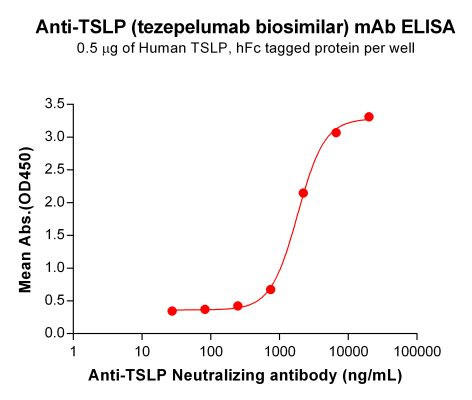
| Tezepelumab (Anti-TSLP) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | TSLP |
| Reactivity: | Human |
How Tezepelumab Biosimilars Support Research
- Cost-Effective Alternative: Allows researchers to study Tezepelumab’s effects without the high costs associated with the original biologic.
- Tool for Drug Development: Helps in screening potential combination therapies and understanding resistance mechanisms.
- Broader Accessibility: Expands research opportunities in institutions with limited funding for biologic studies.
Research Use Only Disclaimer:
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Chris McNally, PhD
Chris McNally, PhD, has a strong foundation in Biomedical Science, completing a PhD scholarship in collaboration with Randox Laboratories and Ulster University. Chris has published extensively in prostate cancer research, focusing on biomarker discovery, cancer risk stratification, and molecular mechanisms such as hypoxia-induced regulation. He currently serves as a Business Development Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Erenumab: Transforming Migraine Prevention Through CGRP Receptor Inhibition
Quick Facts About ErenumabWhat is Erenumab?Erenumab is a fully human monoclonal antibo …1st Apr 2025


