Targeting CD200: Unlocking Immune Suppression in Tumors
Immune evasion is one of the hallmarks of cancer, where tumor cells employ various strategies to suppress immune responses and prevent destruction by the body’s defense systems. CD200, a transmembrane protein, has emerged as a key player in mediating immune suppression in the tumor microenvironment. Targeting CD200 with therapies like OX90 is a promising approach to disrupt this immune evasion, allowing the immune system to mount a more effective attack against cancer cells. This article delves into the role of CD200 in tumors, the therapeutic potential of CD200-targeting agents, and the ongoing research surrounding this pathway.
What is CD200?
CD200, also known as OX2, is a cell surface glycoprotein that belongs to the immunoglobulin superfamily. It is widely expressed on various cell types, including immune cells, endothelial cells, and, importantly, on tumor cells. CD200 interacts with its receptor, CD200R, which is found on immune cells such as macrophages, dendritic cells, and T cells.
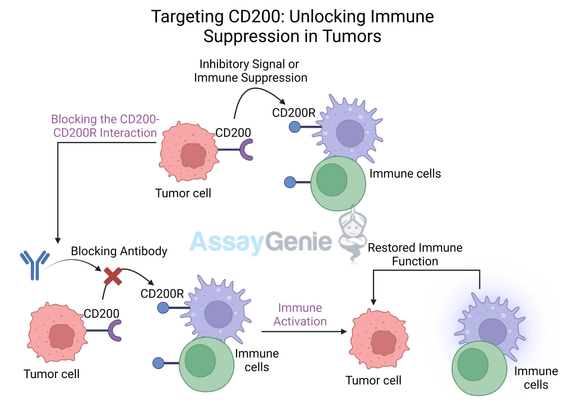
When CD200 binds to CD200R, it delivers an inhibitory signal that reduces the activation and function of these immune cells. This interaction plays a crucial role in maintaining immune tolerance and preventing overactivation of the immune system under normal physiological conditions. However, in the context of cancer, this immune regulation can be co-opted by tumors to suppress anti-tumor immune responses.
CD200 and Tumor Immune Evasion
Tumors often exploit the CD200-CD200R pathway to suppress immune activity within the tumor microenvironment (TME). By overexpressing CD200, tumor cells can inhibit macrophages, dendritic cells, and cytotoxic T cells, reducing the ability of the immune system to recognize and eliminate cancer cells. This mechanism allows tumors to grow and spread unchecked, contributing to immune escape.
Key Mechanism | Role in Tumor Immune Evasion |
|---|---|
CD200-CD200R interaction | Inhibits macrophage, dendritic cell, and T cell activity |
Tumor CD200 overexpression | Suppresses anti-tumor immune responses, promoting immune escape |
Anti-CD200 Therapy: Unlocking the Immune System
Therapies that block CD200, such as OX90, aim to reverse this immune suppression by preventing the interaction between CD200 and CD200R. By targeting this pathway, anti-CD200 antibodies can release the brakes on immune cells, allowing them to become reactivated and attack the tumor more effectively. This strategy represents a new frontier in cancer immunotherapy, focusing on reawakening the immune system's natural ability to fight cancer.
Mechanism of Action: How Anti-CD200 Antibodies Work
Anti-CD200 antibodies, like OX90, bind to CD200 on the surface of tumor cells and block its interaction with CD200R on immune cells. This blockade prevents the inhibitory signal from being delivered to immune cells, allowing them to resume normal function. The result is an increase in immune activation, cytokine production, and the ability of immune cells to target and destroy tumor cells.
Key effects of anti-CD200 antibodies include:
- Restoring immune cell activation: Blocking the CD200-CD200R interaction allows immune cells such as macrophages, dendritic cells, and T cells to become reactivated and resume their anti-tumor functions.
- Enhancing cytokine production: The removal of inhibitory signals leads to increased production of pro-inflammatory cytokines like IFN-γ and TNF-α, which further enhance immune responses against the tumor.
- Boosting T cell-mediated killing: By reactivating T cells, anti-CD200 therapies enhance the immune system’s ability to recognize and destroy tumor cells.
Therapeutic Effect | Outcome |
|---|---|
BlockingCD200-CD200R interaction | Restores immune activation, lifting tumor suppression |
Enhanced cytokine production | Increases pro-inflammatory signaling, improving immune responses |
T cell reactivation | Promotes cytotoxic T cell-mediated tumor destruction |
OX90: A Prominent Anti-CD200 Antibody
OX90 is one of the leading monoclonal antibodies developed to target CD200. By specifically binding to CD200 on the surface of cancer cells, OX90 inhibits the immunosuppressive signals sent to the immune system, promoting the reactivation of immune responses against the tumor. In preclinical studies, OX90 has demonstrated significant anti-tumor effects across a range of cancers, including leukemia, breast cancer, and melanoma.
Enhancing Anti-Tumor Immunity
OX90 has been shown to enhance immune cell infiltration into the tumor microenvironment, promoting the recruitment and activation of effector immune cells. This shift leads to increased tumor destruction and the suppression of tumor growth. OX90 has also shown potential in combination with other immunotherapies, such as checkpoint inhibitors, to further amplify the immune response.
Anti-CD200 Effect | Immune Outcome |
|---|---|
CD200 blockade | Prevents immune suppression within the tumor microenvironment |
Increased immune infiltration | Promotes immune cell recruitment and tumor destruction |
Synergy with Other Immunotherapies
One of the most promising aspects of OX90 and other anti-CD200 therapies is their potential to be combined with existing checkpoint inhibitors like PD-1/PD-L1 and CTLA-4 blockers. Checkpoint inhibitors work by removing inhibitory signals from T cells, unleashing them to attack tumors. However, these therapies may not be fully effective in tumors where the immune system is deeply suppressed by pathways like CD200-CD200R.
By combining anti-CD200 therapy with checkpoint inhibitors, it is possible to create a multi-faceted immune activation, where macrophages, dendritic cells, and T cells are all reactivated to target the tumor. This approach can improve the overall response rate and efficacy of immunotherapies, particularly in immune-resistant cancers.
Combination Therapy | Potential Benefits |
|---|---|
Anti-CD200+ checkpoint inhibitors | Dual blockade of immune suppression, leading to enhanced T cell activation |
Anti-CD200 + chemotherapy | Chemotherapy-induced antigen release, paired with immune reactivation |
Ongoing Clinical Trials and Research
Several clinical trials are underway to evaluate the effectiveness of anti-CD200 therapies like OX90 in cancer patients. These trials are investigating the safety and efficacy of these agents across various cancer types, including hematologic malignancies and solid tumors. Researchers are particularly interested in the potential of anti-CD200 therapies in combination with other treatment modalities, such as chemotherapy, radiation therapy, and other immunotherapies.
Clinical Trial | Cancer Type | Combination Therapy | Phase |
|---|---|---|---|
NCT03022289 | Anti-CD200 monotherapy | Phase I | |
NCT04362703 | Anti- CD200 + checkpoint inhibitors | Phase II | |
NCT04158583 | Anti-CD200 + chemotherapy | Phase I |
CD200 and Immune Cell Reprogramming
Another intriguing area of research is the role of CD200 in macrophage polarization. Macrophages in the tumor microenvironment often adopt an M2 phenotype, which supports tumor growth and suppresses immune responses. Anti-CD200 therapies may help reprogram tumor-associated macrophages (TAMs) from an M2 (pro-tumor) to an M1 (anti-tumor) phenotype, promoting a more aggressive immune attack on the tumor.
Macrophage Phenotype | Function |
|---|---|
Suppresses immune response, supports tumor growth | |
Promotes inflammation, engages in tumor destruction |
Challenges and Future Directions
While anti-CD200 therapies like OX90 offer great potential, there are several challenges to address as these therapies are further developed:
- Tumor heterogeneity: Not all tumors express high levels of CD200, which may limit the effectiveness of CD200-targeting therapies in certain cancers.
- Managing side effects: CD200 is expressed on a variety of normal cells, raising the possibility of off-target effects and immune-related side effects in healthy tissues.
- Optimal combination strategies: Determining the best combinations of anti-CD200 therapies with other cancer treatments, such as checkpoint inhibitors or chemotherapy, is an area of active research.
Future Perspectives
As research continues, anti-CD200 therapies are likely to play an important role in combination with other immunotherapies, particularly in cancers where immune suppression is a dominant feature of the tumor microenvironment. OX90 and other CD200-targeting agents hold promise in unlocking the immune system's ability to fight back against tumors and offer new therapeutic options for patients with immune-resistant cancers.
Conclusion
CD200 represents a critical immune checkpoint that tumors exploit to suppress immune responses and promote survival. By targeting this pathway with therapies like OX90, the immune system can be reactivated, leading to increased tumor clearance and enhanced anti-tumor immunity. As research advances, anti-CD200 therapies may become a key component of immunotherapy, especially when used in combination with other treatments. The ability to unlock immune suppression in tumors offers new hope for improving outcomes in cancer therapy.
References
- Gorczynski, R.M., et al., 2004. CD200 is a potent immunoregulatory molecule and a critical component of the immune
suppressive network in cancer. Clinical Cancer Research, 10(15), pp.4843-4848. - Coles, S.J., et al., 2012. CD200 expression in cancer: a novel means for tumor immune evasion. Blood, 119(15), pp.3567-3576.
- Tonks, A., et al., 2007. The role of CD200 in leukemia and cancer. Blood, 110(8), pp.3822-3831.
- Mahadevan, D., et al., 2010. Targeting CD200 in cancer: A new frontier in immunotherapy. Journal of Clinical Oncology, 28(15), pp.e13048.
- Rygiel, T.P., et al., 2011. CD200-CD200R signaling suppresses anti-tumor immune responses in melanoma. Journal of Immunology, 186(6), pp.4224-4233.
- Barclay, A.N., 2009. Signal regulatory protein alpha (SIRPα): a multi-domain phagocyte signaling receptor. Immunological Reviews, 234(1), pp.75-85.
- Ziegler, S.F., et al., 2013. CD200-CD200R: Immune Regulation and Role in Cancer. Immunology and Cell Biology, 91(3), pp.237-245.
- Jenkinson, W.E., et al., 2015. CD200 and immune suppression in cancer: Emerging therapeutic strategies. Frontiers in Immunology, 6, p.34.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025