Tarextumab: Unlocking the Potential of Cancer Therapeutics
Quick Facts About Tarextumab
What is Tarextumab?
How does Tarextumab work?
Tarextumab inhibits the Notch2/3 receptors, disrupting critical cellular communication pathways in cancer, thereby impeding tumor growth and promoting apoptosis.
What are the clinical applications of Tarextumab?
Tarextumab has been studied in treating pancreatic cancer, non-small cell lung cancer (NSCLC), and small cell lung cancer (SCLC), showcasing its potential in addressing treatment-resistant tumors.
Is Tarextumab safe?
Safety profiles from early trials indicate manageable side effects, with ongoing research focusing on improving therapeutic indices.
1.) Understanding Tarextumab
Tarextumab is a first-in-class monoclonal antibody targeting the Notch2 and Notch3 receptors, critical components of the Notch signaling pathway. This pathway regulates cellular differentiation, proliferation, and survival—processes often exploited in cancer. By selectively inhibiting these receptors, Tarextumab disrupts tumor-stroma interactions that drive cancer progression and resistance to therapy.
Developed by OncoMed Pharmaceuticals, Tarextumab has been extensively studied in preclinical and clinical settings, focusing on its efficacy in solid tumors. Preclinical studies demonstrate its ability to impair tumor growth, reduce stromal support, and enhance the effects of co-administered therapies. Early-phase clinical trials, particularly in pancreatic and lung cancers, highlight its potential to address treatment-resistant malignancies.
A key area of interest is Tarextumab’s role in combination therapies. Preclinical data suggest it can amplify the effects of chemotherapy and immunotherapy by modifying the tumor microenvironment. This disruption of the protective stromal niche enhances immune system recognition of tumor cells and potentiates cytotoxic effects. Tarextumab shows promise in "cold" tumors resistant to standard immunotherapies, positioning it as a valuable agent in the evolving field of immuno-oncology.
With its unique mechanism of action, Tarextumab addresses a critical unmet need in cancer therapy. Ongoing research focuses on optimizing combination strategies, identifying biomarkers for patient selection, and improving dosing regimens to maximize clinical outcomes. Tarextumab’s innovative approach offers hope for overcoming resistance and improving outcomes in advanced and refractory cancers.
2.) Mechanism of Action of Tarextumab
Tarextumab’s mechanism of action is rooted in its targeted inhibition of the Notch signaling pathway, which is often aberrantly activated in cancers. The Notch pathway regulates critical processes such as tumor initiation, angiogenesis, metastasis, and the maintenance of cancer stem cells. By specifically targeting Notch2 and Notch3 receptors, Tarextumab employs a multifaceted approach to combat cancer:
1. Tumor Microenvironment Disruption: Tarextumab disrupts the communication between cancer cells and their surrounding stromal cells, which play a vital role in promoting tumor growth, invasion, and resistance. This disruption weakens the supportive niche that cancer cells rely on, impairing their ability to thrive.
2. Induction of Apoptosis: Many cancer cells are heavily dependent on Notch signaling for survival. By inhibiting this pathway, Tarextumab triggers programmed cell death, or apoptosis, effectively targeting cancer cells that depend on Notch-mediated survival signals.
3. Inhibition of Cancer Stem Cells: The Notch pathway is crucial for the self-renewal and maintenance of cancer stem cells—cells that are resistant to treatment and often responsible for recurrence. Tarextumab reduces the population of these cells, making tumors more vulnerable to therapeutic interventions.
4. Enhanced Chemotherapy Synergy: Preclinical studies demonstrate that Tarextumab enhances the efficacy of chemotherapeutic agents. By sensitizing tumors to cytotoxic effects, it promotes a synergistic relationship that improves treatment outcomes.
Through these mechanisms, Tarextumab addresses key challenges in oncology, including aggressive cancers, resistance, and recurrence, making it a promising candidate for innovative cancer therapies.
3.) Clinical Applications of Tarextumab
Pancreatic Cancer
Pancreatic cancer is one of the most lethal malignancies, with limited treatment options and a poor prognosis. The dense desmoplastic stroma of pancreatic tumors acts as a physical and biochemical barrier, protecting cancer cells and contributing to resistance against therapies. Tarextumab has shown significant promise by targeting the Notch2 and Notch3 receptors involved in maintaining this stroma. Preclinical models demonstrated that Tarextumab disrupts stromal interactions and sensitizes tumors to chemotherapeutic agents. Early-phase clinical trials further suggested that combining Tarextumab with standard-of-care chemotherapy, such as gemcitabine and nab-paclitaxel, could improve progression-free survival and overall outcomes, offering a potential breakthrough for patients with this challenging cancer.
Non-Small Cell Lung Cancer (NSCLC)
Non-small cell lung cancer, which accounts for the majority of lung cancer cases, often exhibits aberrant Notch signaling, driving tumor growth and metastasis. Tarextumab’s ability to inhibit Notch signaling makes it a promising candidate for combating this disease. By impairing tumor-stroma interactions and reducing the survival signals to cancer cells, Tarextumab has shown potential to slow disease progression. Ongoing studies are evaluating its use in combination with other treatments, such as immune checkpoint inhibitors and chemotherapies, to enhance therapeutic efficacy and prolong patient survival.
Small Cell Lung Cancer (SCLC)
Small cell lung cancer is highly aggressive and prone to rapid progression and recurrence. Tarextumab addresses the root cause of this resistance by targeting cancer stem cells, a key driver of SCLC recurrence. Preclinical data suggest that Tarextumab effectively reduces cancer stem cell populations, enhancing response durability and providing a novel approach to addressing treatment-resistant cases. These findings position Tarextumab as a pivotal agent in the fight against SCLC.
4.) Advancing Research with Tarextumab Biosimilar
What is a Biosimilar?
A biosimilar is a biologic product highly similar to an existing reference biologic, with no clinically meaningful differences in safety, purity, or potency. These products play a critical role in making advanced therapies more accessible and affordable for research and development.
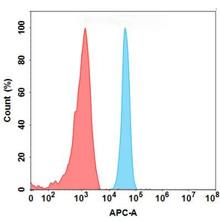
| Tarextumab (Anti-NOTCH3) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | NOTCH3 |
| Reactivity: | Human |
How Tarextumab Biosimilar Compares
The Tarextumab biosimilar retains the core attributes of the original drug while being optimized for research applications. It offers:
- Structural Similarity: Ensures the same binding affinity and specificity for Notch2/3 receptors.
- Cost Efficiency: Reduces financial barriers for exploratory studies and preclinical testing.
- Reliable Supply: Provides consistent quality for large-scale research initiatives.
Benefits in Research
Tarextumab biosimilar accelerates discoveries by enabling researchers to:
- Test novel combinations with emerging therapeutic agents.
- Explore new cancer models, particularly in the context of tumor microenvironment and stem cell biology.
- Conduct high-throughput screening for enhanced drug development.
Research Use Only Disclaimer:
The Tarextumab biosimilar is designated for research use only and not intended for clinical or diagnostic purposes. Its application in preclinical studies continues to drive innovations in cancer therapy.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Miren Ruiz de Eguilaz, PhD
Miren Ruiz de Eguilaz, PhD, has an extensive academic background, earning a BSc in Biology from UPV/EHU, an MSc in Biotechnology from the University of Oviedo, and a PhD in Chemistry from Dublin City University (DCU). Miren’s expertise lies in biosensor technology and bacterial diagnostics. She currently serves as a Product Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



