Sirexatamab: Advancing CD47-Targeted Cancer Therapy and Research
Quick Facts About Sirexatamab
What is Sirexatamab?
How Does Sirexatamab Work?
What Are the Clinical Applications of Sirexatamab?
1.) Understanding Sirexatamab
Sirexatamab is an advanced immunotherapy designed to target CD47, a crucial immune checkpoint often referred to as the "don’t eat me" signal. CD47 is overexpressed in many cancers, allowing tumor cells to escape immune surveillance by inhibiting macrophage-mediated phagocytosis. By blocking CD47, Sirexatamab restores the immune system's ability to recognize and eliminate malignant cells, making it a promising candidate in oncology.
Preclinical and early clinical research has positioned Sirexatamab as a potential breakthrough in cancer treatment. Unlike conventional therapies that solely rely on direct cytotoxicity, this novel approach enhances the body's innate immune response to fight tumors more effectively. It has shown particular promise in combination with existing therapies, including checkpoint inhibitors (such as PD-1/PD-L1 inhibitors) and standard chemotherapies, suggesting it may improve treatment outcomes in patients with resistance to traditional immunotherapy.
While initial trials demonstrated encouraging anti-tumor activity, ongoing studies are focused on optimizing dosing strategies, minimizing immune-related adverse events, and identifying patient subgroups that would benefit most. One of the key challenges in CD47-targeting therapies is managing off-target effects, as CD47 is also present on healthy cells, including red blood cells. Researchers are actively working on strategies such as lower dosing, intermittent treatment schedules, and bispecific antibody designs to enhance specificity and reduce toxicity.
The future of Sirexatamab in clinical oncology remains promising, with further investigations exploring its efficacy in both solid and hematologic malignancies. As research advances, Sirexatamab could emerge as a key component in next-generation immunotherapy regimens, transforming the landscape of cancer treatment by leveraging the immune system’s natural ability to combat disease.
2.) Mechanism of Action of Sirexatamab
Sirexatamab exerts its therapeutic effects by blocking the interaction between CD47 and its ligand, signal regulatory protein alpha (SIRPα), which is primarily found on macrophages and dendritic cells. Under normal conditions, the CD47-SIRPα interaction transmits an inhibitory signal that prevents macrophages from engulfing and destroying cells, including cancerous ones. By disrupting this signaling pathway, Sirexatamab removes the “don’t eat me” signal, allowing macrophages to recognize, engulf, and eliminate tumor cells through phagocytosis.
Preclinical studies have shown that Sirexatamab significantly enhances macrophage-mediated clearance of malignant cells. It also stimulates dendritic cells, leading to antigen presentation and the activation of adaptive immune responses, further amplifying anti-tumor immunity. Notably, Sirexatamab has demonstrated synergy with checkpoint inhibitors (such as PD-1/PD-L1 inhibitors) by promoting a more comprehensive immune response, making it a valuable candidate for combination therapies.
One of the challenges associated with CD47-targeting therapies is their potential for on-target toxicity. Since CD47 is expressed on normal hematopoietic cells, including red blood cells, its inhibition can lead to side effects such as anemia. To mitigate these risks, researchers are exploring alternative dosing regimens, Fc region modifications to reduce red blood cell interactions, and combination strategies that minimize toxicity while maintaining efficacy.
As research continues, scientists are also investigating the potential for Sirexatamab in treating non-cancerous conditions where immune evasion plays a role, such as fibrosis and autoimmune diseases. The ability to fine-tune its effects while reducing unintended immune activation remains a key area of exploration. With ongoing refinements, Sirexatamab represents a promising therapeutic approach with the potential to reshape cancer treatment paradigms.
3.) Clinical Applications of Sirexatamab
Sirexatamab has been investigated in multiple clinical trials for its potential to treat both hematologic and solid tumors. Given its mechanism of action, it is particularly relevant in cancers that exhibit immune evasion through CD47 overexpression, such as non-Hodgkin lymphoma, acute myeloid leukemia (AML), and solid tumors like ovarian and colorectal cancers.
In hematologic malignancies, studies have suggested that Sirexatamab enhances responses to standard-of-care treatments, such as chemotherapy and targeted therapies. For instance, in AML, combining Sirexatamab with azacitidine has shown promising preliminary results in improving remission rates. Similarly, in lymphomas, the therapy has demonstrated the ability to work alongside rituximab to boost tumor cell clearance. These findings indicate that Sirexatamab could become a key player in addressing treatment resistance and improving long-term outcomes.
For solid tumors, early clinical trials have explored its efficacy as a monotherapy and in combination with immune checkpoint inhibitors. Since tumors in pancreatic, ovarian, and colorectal cancers frequently overexpress CD47, Sirexatamab's ability to enhance macrophage and dendritic cell activity may provide a new therapeutic avenue for these difficult-to-treat cancers. The drug has also been tested alongside PD-1/PD-L1 inhibitors, showing potential in overcoming resistance mechanisms that limit the success of conventional immunotherapy.
Ongoing research aims to refine patient selection criteria to identify which populations are most likely to benefit from CD47 blockade. Additionally, strategies are being developed to optimize dosing regimens and manage immune-related side effects. While challenges remain, including potential hematologic toxicities, continued advancements in antibody engineering and biomarker-driven patient selection will likely enhance Sirexatamab’s clinical impact.
As research progresses, Sirexatamab could play a critical role in future immunotherapy protocols, broadening treatment options for patients with difficult-to-treat cancers and potentially leading to durable remissions in cases where conventional therapies fall short.
4.) Exploring Biosimilars for Sirexatamab
What is a Biosimilar?
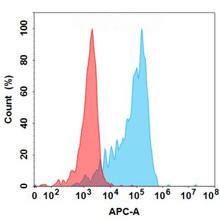
| Sirexatamab (Anti-DKK1) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | DKK1 |
| Reactivity: | Human |
How Sirexatamab Biosimilar Compares to Sirexatamab
Benefits of Sirexatamab Biosimilar
Research Use Only Disclaimer:
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Marina Alberto, PhD
Marina Alberto, PhD, holds a robust academic background in Biotechnology, earning her Bachelor’s Degree and PhD in Science and Technology from Quilmes National University. Her research spans cancer immunotherapy, glycan profiling, and vaccine development, including innovative projects on pediatric leukemia diagnosis and cancer-associated carbohydrate-mimetic vaccines. She currently serves as a Technical Support and Sales Specialist at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



