Rovalpituzumab Tesirine: Advancing Small Cell Lung Cancer Research
Quick Facts About Rovalpituzumab Tesirine
What is Rovalpituzumab Tesirine?
Rovalpituzumab Tesirine (Rova-T) is an antibody-drug conjugate (ADC) designed to target DLL3, a protein overexpressed in small cell lung cancer (SCLC) cells.
How does Rovalpituzumab Tesirine work?
It binds to DLL3, delivering a cytotoxic agent that induces tumor cell death while sparing healthy cells.
What are the clinical applications of Rovalpituzumab Tesirine?
It has been studied in SCLC and other DLL3-expressing cancers, with research focused on improving efficacy and reducing toxicity.
1.) Understanding Rovalpituzumab Tesirine
Rovalpituzumab Tesirine, commonly referred to as Rova-T, is an investigational antibody-drug conjugate (ADC) specifically developed to target Delta-like protein 3 (DLL3). DLL3 is highly expressed in small cell lung cancer (SCLC) but is largely absent in normal tissues, making it a promising target for precision oncology. The development of Rova-T was driven by the need for innovative treatment options in SCLC, a highly aggressive malignancy with poor prognosis and limited effective therapies.
Unlike conventional chemotherapy, which broadly attacks dividing cells, Rova-T delivers a cytotoxic payload directly to cancer cells expressing DLL3. This is achieved through a monoclonal antibody that specifically binds to DLL3, ensuring targeted delivery of its potent cytotoxic agent, a pyrrolobenzodiazepine (PBD) dimer toxin. PBD dimers are known for their ability to induce irreversible DNA damage, leading to apoptosis in tumor cells while minimizing damage to surrounding healthy tissue.
Early clinical trials demonstrated promising antitumor activity, generating optimism for Rova-T’s potential as a breakthrough therapy in SCLC. However, subsequent studies raised concerns regarding its safety profile. High toxicity levels, particularly severe treatment-related adverse effects, led to the discontinuation of certain trials and regulatory setbacks. Despite these challenges, Rova-T’s foundational research has significantly contributed to the ongoing development of DLL3-targeting therapies.
Scientists are now exploring next-generation ADCs, improved drug-linker technologies, and combination strategies to optimize the therapeutic window and enhance safety while maintaining efficacy. Rova-T’s legacy in targeted therapy research continues to influence new approaches aimed at overcoming the limitations seen in early DLL3-targeting treatments, offering hope for improved outcomes in SCLC and other hard-to-treat malignancies.
2.) Mechanism of Action of Rovalpituzumab Tesirine
Rovalpituzumab Tesirine operates through a highly specific and targeted mechanism designed to exploit the tumor-associated expression of DLL3. The process involves several key steps that enable precise drug delivery while minimizing systemic toxicity.
1. Targeting DLL3: The monoclonal antibody component of Rova-T selectively binds to DLL3, a transmembrane protein expressed on the surface of SCLC cells but absent in normal tissues. This ensures that the cytotoxic payload is directed exclusively toward malignant cells.
2. Internalization and Drug Release: Once bound to DLL3, the ADC-receptor complex undergoes internalization into the cancer cell. Inside the cell, lysosomal degradation triggers the release of the cytotoxic payload, a pyrrolobenzodiazepine (PBD) dimer toxin.
3. DNA Damage and Apoptosis: The released PBD toxin functions as a DNA cross-linking agent, inducing irreparable DNA strand breaks. This leads to cell cycle arrest and apoptosis, effectively eliminating the tumor cell.
4. Bystander Effect: One of the unique properties of the PBD payload is its ability to diffuse into nearby cells, potentially eliminating adjacent DLL3-expressing tumor cells. This bystander effect enhances the therapeutic potential but may also contribute to toxicity concerns.
While Rova-T’s mechanism is highly targeted, challenges such as dose-limiting toxicities and unintended off-target effects have necessitated further refinement. Researchers are now investigating improved linker technologies, alternative cytotoxic agents, and combination regimens incorporating immune checkpoint inhibitors to maximize efficacy and safety. The insights gained from Rova-T’s mechanism continue to inform the development of next-generation ADCs, reinforcing the importance of DLL3 as a viable target in oncology.
3.) Clinical Applications of Rovalpituzumab Tesirine
Rovalpituzumab Tesirine has been primarily explored as a potential therapy for small cell lung cancer (SCLC), a malignancy characterized by rapid tumor growth, early metastasis, and a poor survival rate. Given that DLL3 is overexpressed in more than 80% of SCLC tumors but absent in normal tissue, Rova-T was designed to serve as a precision medicine approach for this aggressive cancer type.
1. Small Cell Lung Cancer (SCLC): SCLC remains one of the most challenging cancers to treat, with chemotherapy and radiation therapy offering limited long-term benefits. Rova-T was initially positioned as a novel therapeutic strategy to improve outcomes in DLL3-positive SCLC patients. Early-phase clinical trials indicated encouraging responses, sparking hope for a new targeted treatment. However, subsequent studies revealed substantial toxicity concerns, including severe adverse events such as pleural effusion and peripheral edema, leading to the discontinuation of some pivotal trials. Despite these hurdles, the concept of DLL3-targeting remains a focal point in current SCLC research.
2. Combination Therapies: The development setbacks of Rova-T have spurred new investigations into combination strategies. Researchers are exploring the integration of DLL3-targeting ADCs with immune checkpoint inhibitors, chemotherapy, or other targeted agents to enhance efficacy while mitigating toxicity. These approaches aim to harness immune modulation and optimize tumor cell eradication while reducing systemic side effects.
3. Future Directions: Although Rova-T itself is no longer in active clinical development, its foundational research continues to influence the next generation of targeted therapies. Innovations in ADC technology, such as more stable linker systems and novel cytotoxic payloads, are being incorporated into new DLL3-targeting candidates. Additionally, the exploration of DLL3 as a biomarker for precision oncology is expanding beyond SCLC, with potential implications for neuroendocrine tumors and other DLL3-expressing malignancies.
The lessons learned from Rova-T’s clinical journey underscore the complexity of targeted ADC therapy and highlight the need for ongoing refinement in drug design and patient selection. While its clinical application as a standalone agent faced challenges, Rova-T’s contributions continue to shape the evolving landscape of precision oncology, offering valuable insights for the future of DLL3-targeted therapeutics.
4.) Exploring Biosimilars for Rovalpituzumab Tesirine
As research into DLL3-targeting therapies progresses, biosimilars play a crucial role in furthering scientific discovery.
What is a Biosimilar?
A biosimilar is a biologic drug designed to be highly similar to an existing approved biologic therapy. These products provide researchers with cost-effective options for preclinical and translational studies.
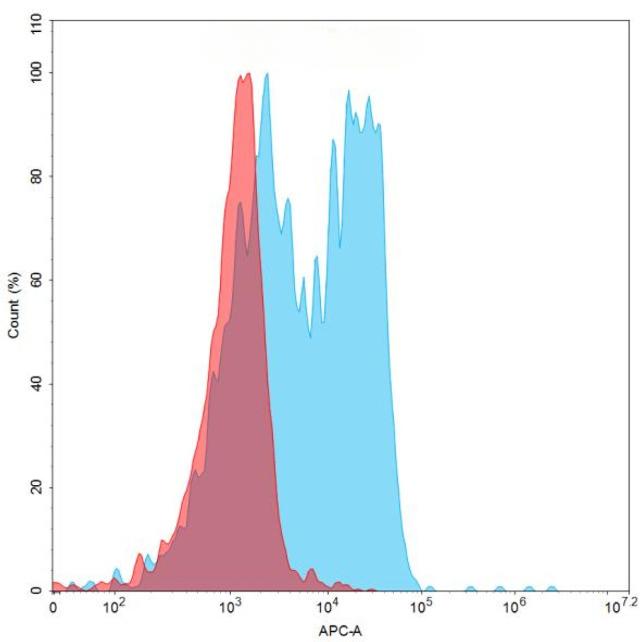
| Rovalpituzumab (Anti-DLL3) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | DLL3 |
| Reactivity: | Human |
Comparison of Rovalpituzumab Tesirine and Its Biosimilar
Biosimilars maintain the core characteristics of the original biologic while offering accessibility advantages for research. Key similarities and differences include:
- Mechanism of Action: Both target DLL3 and deliver cytotoxic payloads to tumor cells.
- Research Applications: Biosimilars enable the study of DLL3-targeting mechanisms without the limitations of clinical-grade production constraints.
- Safety and Efficacy Considerations: While biosimilars are not intended for direct patient use, they facilitate early-stage drug development and experimental therapies.
Benefits of Biosimilars in Research
2. Cost-Effective Solutions: Enables expanded preclinical studies without the financial burden of clinical-grade biologics.
2. Facilitates Innovation: Helps refine future ADC designs and combination strategies.Research Use Only Disclaimer:
Biosimilars of Rovalpituzumab Tesirine are for research purposes only and are not intended for human therapeutic use.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Marina Alberto, PhD
Marina Alberto, PhD, holds a robust academic background in Biotechnology, earning her Bachelor’s Degree and PhD in Science and Technology from Quilmes National University. Her research spans cancer immunotherapy, glycan profiling, and vaccine development, including innovative projects on pediatric leukemia diagnosis and cancer-associated carbohydrate-mimetic vaccines. She currently serves as a Technical Support and Sales Specialist at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



