Rosopatamab: Unveiling the Potential of Anti-CD47 Therapy in Cancer Research
Quick Facts About Rosopatamab
What is Rosopatamab?
What is the mechanism of action for Rosopatamab?
What are the clinical applications of Rosopatamab?
1.) Understanding Rosopatamab
Rosopatamab is an innovative monoclonal antibody designed to target CD47, a key immune checkpoint protein often overexpressed in cancer cells. CD47 functions as a "don't eat me" signal, helping tumor cells evade immune surveillance by preventing macrophages and dendritic cells from attacking them. Many aggressive malignancies, including hematologic cancers like leukemia and lymphoma, as well as solid tumors such as ovarian and lung cancers, utilize this pathway to persist and spread. By blocking CD47, Rosopatamab restores the ability of the immune system to recognize and destroy cancerous cells, making it a promising candidate in immuno-oncology.
One of the most significant challenges in CD47-targeting therapies, including Rosopatamab, is minimizing off-target effects. Since CD47 is also expressed on normal cells, particularly red blood cells, blocking it can lead to unintended consequences like anemia or excessive immune activation. Researchers are actively exploring ways to optimize Rosopatamab’s specificity, ensuring that only tumor cells are targeted while preserving healthy cells.
Early studies suggest that Rosopatamab has strong potential as a monotherapy and in combination with other immune-based treatments. Scientists are investigating its ability to work synergistically with checkpoint inhibitors, chemotherapies, and radiotherapies, enhancing the immune system’s ability to eradicate cancer. While some CD47 inhibitors have encountered challenges, including toxicity and compensatory immune mechanisms, ongoing research aims to refine Rosopatamab’s efficacy. Advanced formulations and novel drug delivery methods are being explored to mitigate side effects and improve therapeutic outcomes.
Given its potential, Rosopatamab is a subject of intense clinical interest. Researchers are studying its role in various cancers, with clinical trials evaluating its safety, dosing, and effectiveness in different patient populations. If successfully developed, it could revolutionize how immune checkpoint therapy is applied across oncology, paving the way for more effective, less toxic cancer treatments.
2.) Mechanism of Action of Rosopatamab
Rosopatamab exerts its therapeutic effect by inhibiting CD47, a transmembrane protein that interacts with SIRPα (Signal Regulatory Protein Alpha) on macrophages. This interaction normally prevents immune cells from attacking CD47-expressing cells, acting as a crucial self-recognition signal to protect normal tissues. However, cancer cells exploit this mechanism by overexpressing CD47, allowing them to evade immune destruction.
By blocking CD47, Rosopatamab disrupts this immune evasion strategy, reactivating the body’s natural defenses. Macrophages can now identify and engulf tumor cells through phagocytosis, preventing the cancer from proliferating. This process is particularly effective in blood cancers such as non-Hodgkin lymphoma and acute myeloid leukemia (AML), where CD47 expression is especially high. However, the benefits extend to solid tumors as well, as researchers have found that CD47 blockade enhances immune-mediated tumor clearance.
A key advantage of Rosopatamab over conventional chemotherapy is its targeted approach. Unlike cytotoxic drugs that indiscriminately kill both healthy and cancerous cells, Rosopatamab works by amplifying the body’s existing immune response, leading to potentially lower toxicity and fewer side effects. Moreover, Rosopatamab’s mode of action is complementary to other immunotherapies like PD-1/PD-L1 inhibitors, which target adaptive immune checkpoints. In combination therapy, CD47 blockade enhances the efficacy of checkpoint inhibitors, leading to more robust and sustained anti-tumor activity.
Despite its promise, CD47-targeting therapies, including Rosopatamab, face significant challenges. One of the primary concerns is that red blood cells also express CD47, leading to possible anemia and hematologic toxicities. To address this, researchers are working on modified drug formulations and optimized dosing schedules that selectively target cancerous tissues while sparing normal cells. Advanced strategies, such as engineering Rosopatamab to have a stronger affinity for CD47 in the tumor microenvironment, are currently under investigation.
3.) Clinical Applications of Rosopatamab
Rosopatamab is currently being investigated in various clinical settings, with a primary focus on hematologic malignancies and solid tumors. Due to its mechanism of blocking CD47 and enhancing macrophage-mediated phagocytosis, this monoclonal antibody has the potential to improve outcomes in patients with cancers that have limited treatment options.
One of the most promising areas of research is its application in hematologic cancers. Studies indicate that acute myeloid leukemia (AML), non-Hodgkin lymphoma (NHL), and multiple myeloma frequently overexpress CD47, making them ideal candidates for CD47-blocking therapies. Early clinical trials suggest that Rosopatamab could enhance tumor clearance and improve survival rates when combined with standard treatments like rituximab (a CD20-targeting antibody) or chemotherapy regimens.
Beyond blood cancers, solid tumors such as ovarian cancer, non-small cell lung cancer (NSCLC), and pancreatic cancer are also being explored in clinical trials. These cancers often exhibit high CD47 expression, allowing them to evade immune detection. By blocking CD47, Rosopatamab may restore immune surveillance, making these tumors more susceptible to attack by macrophages and other immune cells.
A key area of investigation is combination therapy. Many researchers believe that Rosopatamab’s efficacy can be significantly enhanced when paired with other immune checkpoint inhibitors, such as PD-1/PD-L1 inhibitors like pembrolizumab or nivolumab. The rationale is that CD47 blockade activates innate immunity (macrophages and dendritic cells), while PD-1 blockade stimulates adaptive immunity (T cells), leading to a more comprehensive immune attack on tumors. Preclinical studies suggest that this combination could lead to higher response rates and prolonged remission in cancer patients.
Despite its potential, there are challenges in clinical development. One of the main hurdles is the risk of anemia due to CD47’s expression on red blood cells. However, researchers are optimizing dosing strategies and drug formulations to mitigate this issue. Additionally, identifying biomarkers to predict patient response is another priority, as not all tumors may be equally susceptible to CD47 blockade.
4.) Exploring Biosimilars for Rosopatamab
What is a Biosimilar?
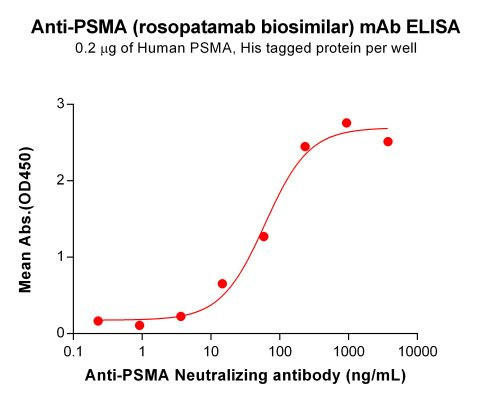
| Rosopatamab (Anti-PSMA) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | PSMA |
| Reactivity: | Human |
How Rosopatamab Biosimilar Compares to Rosopatamab
Advancing Research on Rosopatamab
Research Use Only Disclaimer:
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By David Lee, PhD
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



