Plonmarlimab: Advancing Anti-GM-CSF Therapy in Inflammatory Diseases
Quick Facts About Plonmarlimab
What is Plonmarlimab?
How does Plonmarlimab work?
What are the clinical applications of Plonmarlimab?
Is Plonmarlimab safe?
1.) Understanding Plonmarlimab
Plonmarlimab is a humanized monoclonal antibody specifically designed to neutralize granulocyte-macrophage colony-stimulating factor (GM-CSF), a cytokine that plays a critical role in immune system regulation. GM-CSF is responsible for stimulating the production and activation of macrophages, neutrophils, and dendritic cells, which are essential components of the immune response. However, excessive GM-CSF signaling has been linked to various inflammatory and autoimmune diseases, where unchecked immune activation leads to chronic inflammation and tissue damage.
By blocking GM-CSF, Plonmarlimab aims to reduce pathological inflammation while preserving normal immune function. This approach has gained interest as an alternative to traditional immunosuppressants, which often carry significant side effects, such as increased infection risk and broad immune suppression. Plonmarlimab represents a more targeted strategy, focusing on a key inflammatory pathway rather than broadly dampening the immune system.
Recent studies have explored its potential for treating rheumatoid arthritis (RA), cytokine release syndromes (CRS), and other immune-driven disorders. One of its most notable applications has been in COVID-19-associated hyperinflammation, where excessive immune activation leads to severe complications, including acute respiratory distress syndrome (ARDS). Researchers have investigated whether GM-CSF blockade could mitigate these life-threatening inflammatory responses.
2.) Mechanism of Action of Plonmarlimab
Plonmarlimab exerts its therapeutic effect by binding to GM-CSF, preventing it from interacting with its receptors on myeloid immune cells, including macrophages, neutrophils, and dendritic cells. GM-CSF signaling is crucial for the activation and differentiation of these cells, leading to the production of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1 beta (IL-1β). These cytokines play a pivotal role in the progression of autoimmune and inflammatory diseases, contributing to tissue destruction and chronic inflammation.
By blocking GM-CSF signaling, Plonmarlimab effectively reduces the activation of these immune cells, leading to lower cytokine production and decreased inflammation. This targeted inhibition is particularly beneficial in conditions like rheumatoid arthritis, systemic inflammatory diseases, and cytokine storm syndromes, where excessive immune responses result in significant morbidity. Unlike broad-spectrum immunosuppressants, which can increase susceptibility to infections, Plonmarlimab specifically modulates a single inflammatory pathway, offering a more precise therapeutic approach.
One of the major challenges in targeting GM-CSF is the potential impact on normal immune homeostasis. GM-CSF is also essential for lung surfactant production and innate immune defense, meaning its long-term inhibition could pose risks. To address this, researchers are working to refine dosing regimens and identify biomarkers that predict which patients are most likely to benefit from treatment without experiencing adverse effects.
3.) Clinical Applications of Plonmarlimab
Plonmarlimab has been investigated in various clinical settings, particularly in diseases characterized by excessive immune activation and chronic inflammation. Its primary areas of research include rheumatoid arthritis (RA), cytokine release syndrome (CRS), and other autoimmune conditions where conventional therapies have limitations.
One of the most promising applications of Plonmarlimab is in the treatment of rheumatoid arthritis (RA), a chronic autoimmune disease in which immune cells attack the joints, leading to pain, swelling, and disability. Studies suggest that GM-CSF blockade can help reduce inflammation and slow disease progression, providing an alternative for patients who do not respond to traditional disease-modifying antirheumatic drugs (DMARDs) or biologics targeting TNF-α and IL-6.
Additionally, Plonmarlimab was explored as a potential treatment for COVID-19-associated hyperinflammation, specifically in cases of cytokine storm syndrome. In severe COVID-19 infections, an overactive immune response can cause multi-organ failure and acute respiratory distress syndrome (ARDS). By inhibiting GM-CSF, researchers hoped to mitigate uncontrolled inflammation and improve survival rates in critically ill patients. However, clinical trials yielded mixed results, leading to some studies being discontinued due to insufficient efficacy in specific patient populations.
Beyond RA and COVID-19, ongoing investigations are assessing the effectiveness of Plonmarlimab in other immune-mediated diseases, including multiple sclerosis (MS), systemic lupus erythematosus (SLE), and inflammatory lung diseases. While some trials have been halted, the data collected continues to inform the development of future GM-CSF inhibitors, refining therapeutic approaches in autoimmune diseases.
4.) Exploring Biosimilars for Plonmarlimab
What is a Biosimilar?
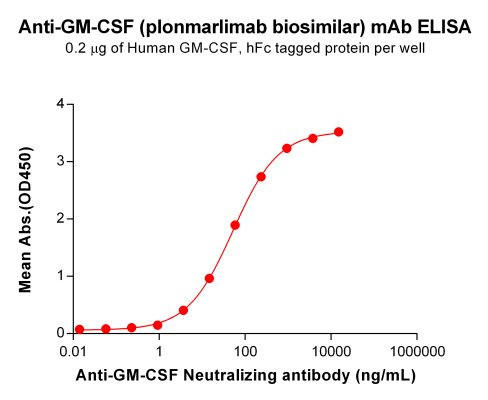
| Plonmarlimab (Anti-GM-CSF) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | GM-CSF |
| Reactivity: | Human |
How Plonmarlimab Biosimilar Compares to Plonmarlimab
Benefits of Plonmarlimab Biosimilar in Research
Research Use Only Disclaimer:
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By David Lee, PhD
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



