Patritumab Deruxtecan: Mechanism, Clinical Applications, and Biosimilar Advances
Quick Facts About Patritumab Deruxtecan
What is Patritumab Deruxtecan?
Patritumab Deruxtecan (HER3-DXd) is an investigational antibody-drug conjugate (ADC) targeting HER3, a receptor implicated in various cancers, including non-small cell lung cancer (NSCLC) and breast cancer.
How Does Patritumab Deruxtecan Work?
This ADC binds to HER3-expressing tumor cells, delivering a topoisomerase I inhibitor payload that induces targeted cell death.
What Are the Clinical Applications of Patritumab Deruxtecan?
Emerging research explores its efficacy in NSCLC, breast cancer, and other HER3-expressing malignancies. Recent trials focus on its potential in overcoming resistance to standard therapies.
Is Patritumab Deruxtecan Approved by the FDA?
As of recent updates, Patritumab Deruxtecan is under investigation, with regulatory discussions ongoing. Stay tuned for the latest developments.
1.) Understanding Patritumab Deruxtecan
Patritumab Deruxtecan (HER3-DXd) represents a cutting-edge advancement in targeted cancer therapy, focusing on HER3 as a precision target for drug delivery. Unlike traditional chemotherapy, which indiscriminately affects both healthy and malignant cells, this antibody-drug conjugate (ADC) selectively binds to HER3-expressing tumor cells, ensuring a more concentrated and effective treatment approach. While HER3 was once considered a secondary target, recent research has unveiled its crucial role in tumor proliferation, metastasis, and resistance to existing therapies, making it an increasingly important therapeutic target.
Developed by Daiichi Sankyo in collaboration with AstraZeneca, Patritumab Deruxtecan is part of a new generation of ADCs designed to maximize therapeutic efficacy while minimizing systemic toxicity. Overexpression of HER3 has been observed in various treatment-resistant cancers, such as non-small cell lung cancer (NSCLC) and breast cancer, positioning it as a highly attractive target for intervention. This specificity allows Patritumab Deruxtecan to address a significant unmet need in oncology, particularly for patients who have exhausted conventional treatments like chemotherapy and tyrosine kinase inhibitors (TKIs).
Preclinical studies and early-phase clinical trials have demonstrated promising anti-tumor activity, particularly in lung and breast cancers with high HER3 expression. The investigational drug has shown efficacy in targeting resistant tumors, offering hope for patients with limited treatment options. Ongoing research is focused on optimizing dosing strategies, evaluating long-term safety, and exploring the potential for combining Patritumab Deruxtecan with other targeted therapies. If successfully developed, it could reshape the treatment landscape for HER3-positive malignancies, offering a novel therapeutic avenue for patients facing challenging cancers.
2.) Mechanism of Action of Patritumab Deruxtecan
Patritumab Deruxtecan is designed to selectively target HER3-expressing cancer cells, delivering a potent cytotoxic payload with precision. Its mechanism of action consists of three key steps:
1. Targeting HER3
The monoclonal antibody component of Patritumab Deruxtecan binds to HER3 receptors on tumor cells, ensuring specificity. HER3 is frequently overexpressed in resistant tumors, making it an optimal target for ADC therapy.
2. Internalization and Payload Release
Upon binding to HER3, the ADC undergoes receptor-mediated endocytosis, allowing the entire drug complex to be internalized by the cancer cell. Once inside, the linker connecting the antibody to the cytotoxic payload is cleaved, releasing the active drug within the tumor cell.
3. DNA Damage and Apoptosis
The released payload, a potent topoisomerase I inhibitor, disrupts DNA replication, leading to irreparable DNA damage and ultimately inducing tumor cell apoptosis. This targeted delivery mechanism ensures maximum tumor cell death while minimizing toxicity to surrounding healthy tissues.
Unlike conventional chemotherapy, which affects both healthy and malignant cells, Patritumab Deruxtecan provides a more controlled and efficient approach to cancer treatment. By selectively targeting HER3-expressing tumor cells, this antibody-drug conjugate (ADC) ensures that the therapeutic payload is delivered directly to the tumor, enhancing anti-tumor activity while minimizing systemic side effects. This targeted mechanism reduces damage to healthy tissues, a common concern with traditional chemotherapy. Ongoing clinical trials are further exploring Patritumab Deruxtecan’s role in different cancer subtypes, assessing its effectiveness across various HER3-positive tumors, and investigating its potential synergy when combined with other immunotherapies and targeted treatments.
3.) Clinical Applications of Patritumab Deruxtecan
Patritumab Deruxtecan has shown significant promise in treating HER3-positive malignancies, particularly in lung and breast cancers. Its key applications include:
1. Non-Small Cell Lung Cancer (NSCLC)
HER3 expression is prevalent in NSCLC, particularly in EGFR-mutated cases where patients develop resistance to tyrosine kinase inhibitors (TKIs). Preliminary studies indicate that Patritumab Deruxtecan may effectively target these resistant cancer cells, offering a new line of treatment for patients who have exhausted standard therapies.
2. Breast Cancer
HER3 expression is also common in breast cancer, especially in hormone receptor-positive and triple-negative subtypes. Preclinical and early-phase clinical trials suggest that Patritumab Deruxtecan may enhance treatment responses in these patients, either as a monotherapy or in combination with other targeted agents.
3. Other Solid Tumors
Beyond lung and breast cancers, Patritumab Deruxtecan is being investigated for its efficacy in head and neck squamous cell carcinoma and other HER3-expressing malignancies. Researchers are exploring its potential as part of combination therapy strategies to overcome drug resistance and enhance clinical outcomes.
While further data is needed, initial clinical findings suggest that Patritumab Deruxtecan may offer an effective alternative for patients with limited treatment options. Its ability to deliver a highly potent cytotoxic agent directly to tumor cells while minimizing systemic toxicity represents a significant advancement in precision oncology.
4.) Exploring Biosimilars for Patritumab Deruxtecan
What is a Biosimilar?
A biosimilar is a biologic product highly similar to an approved reference biologic, with no clinically meaningful differences in efficacy or safety. Biosimilars play a crucial role in expanding research opportunities and improving drug accessibility.
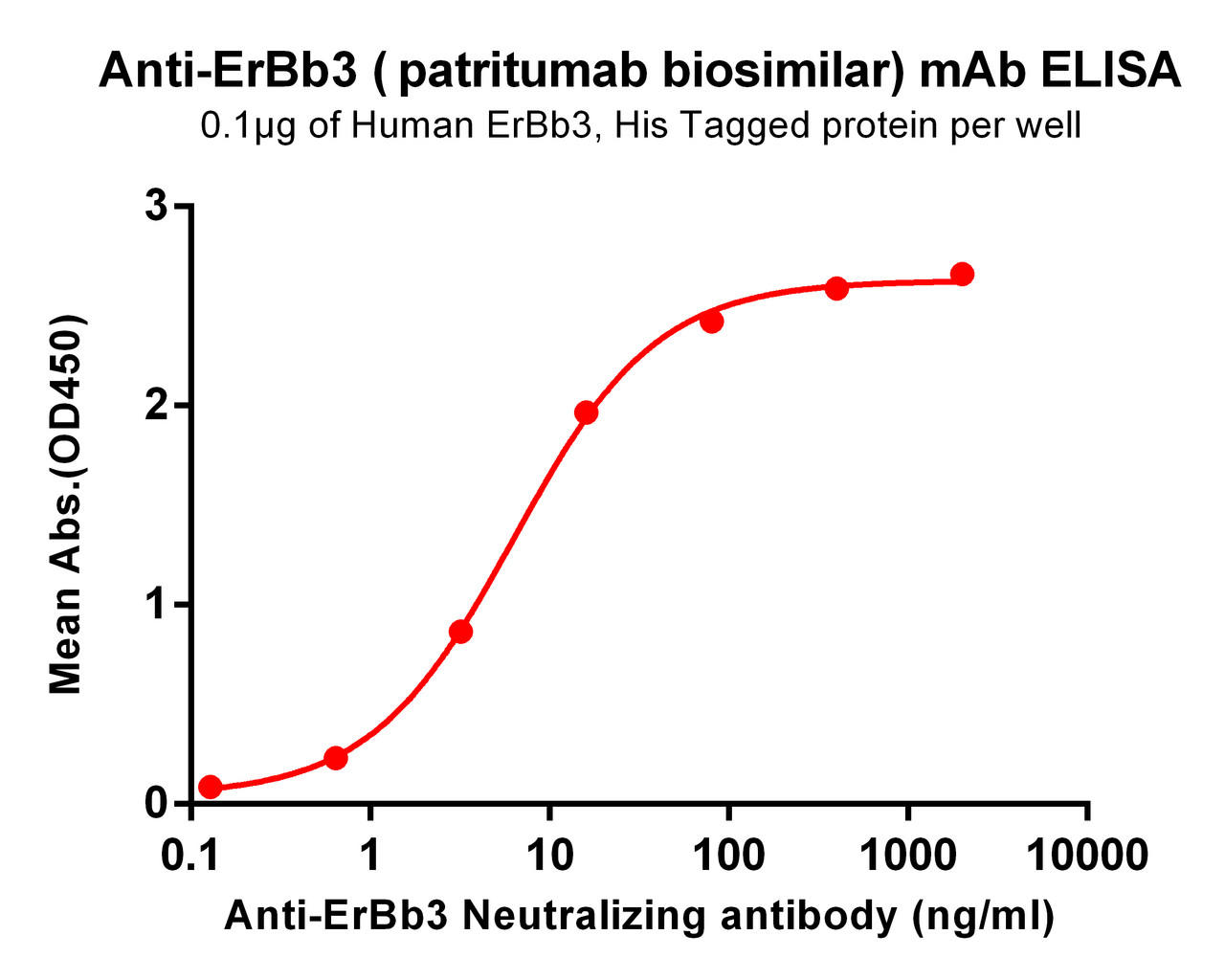
| Patritumab (Anti-HER3) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | HER-3 |
| Reactivity: | Human |
Patritumab Biosimilar: Advancing Research
Patritumab biosimilars serve as valuable tools for studying HER3-targeted therapies. By offering a research-grade alternative, biosimilars facilitate:
- Preclinical Investigations – Enabling drug interaction and resistance mechanism studies.
- Cost-Effective Research – Providing an accessible option for academic and pharmaceutical research.
- Broader Access to Biologic Studies – Supporting innovative drug development approaches.
Comparison with Patritumab Deruxtecan
While the biosimilar is not a direct therapeutic replacement, it provides structural and functional similarities that make it a powerful research asset. Differences include:
- Intended Use: Research-focused rather than clinical application.
- Manufacturing Process: Unique production methodologies that meet research-specific standards.
Research Use Only Disclaimer:
Patritumab biosimilars are intended for research purposes only and are not approved for clinical use. Their role is to support ongoing discoveries and drug development initiatives.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Marina Alberto, PhD
Marina Alberto, PhD, holds a robust academic background in Biotechnology, earning her Bachelor’s Degree and PhD in Science and Technology from Quilmes National University. Her research spans cancer immunotherapy, glycan profiling, and vaccine development, including innovative projects on pediatric leukemia diagnosis and cancer-associated carbohydrate-mimetic vaccines. She currently serves as a Technical Support and Sales Specialist at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



