Neurofilament Light chain (NEFL) as a biomarker for neuronal damage
Delve into the pivotal role of Neurofilament Light chain (NEFL) in neuron structure and its emerging importance as a biomarker for various neurodegenerative diseases, offering insights into disease progression and treatment monitoring.
Key Takeaways:
- Explore the significance of Neurofilament Light chain (NEFL) as a biomarker for neuronal damage and its role in neurodegenerative diseases.
- Understand NEFL's function in the neuronal cytoskeleton, transport mechanisms, and post-translational modifications.
- Discover NEFL's involvement in conditions like Alzheimer’s, ALS, Huntington’s disease, and its potential as a diagnostic tool.
Neurofilament Light Chain (NEFL)
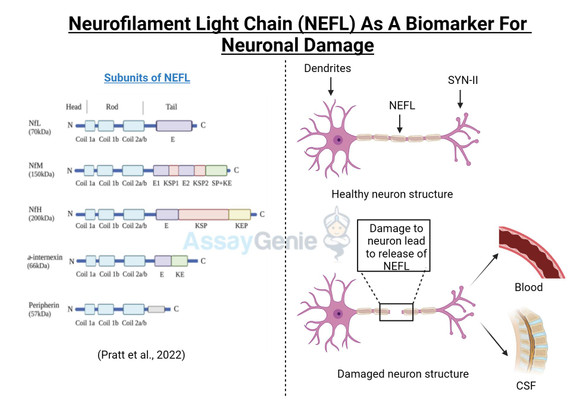
Neurofilament Light chain (NEFL, or NfL) is one of three subunits of the neurofilament filaments, which are intermediate filament proteins expressed in neurons that play a central role in the transport of molecules throughout the axon, and contribute to the integrity of the neuronal cytoarchitechture. The neuronal cytoskeleton contains several filaments, classified as actin microfilaments, microtubules, and intermediate filaments (Ishikawa et al, 19680. Furthermore, two main families of intermediate filaments are expressed in the Central and Peripheral Nervous System (CNS, PNS); glial filaments, such as GFAP and vimentin; and neurofilaments, which have a diameter of approximately 10 nm (Cardone and Roots, 1990; Karlsson et al, 1987). Neurofilaments are further made up of 3 subunits; a heavy chain, a middle chain and a light chain, based on their molecular weights, 112.5 kDa, 102.5 kDa, and 61.5 kDa, respectively; although they display higher molecular weights on polyacrylamide gels, due to the high content of negatively charged amino acids in their structure, reaching 205 kDa, 168 kDa, and 68 kDa respectively (Julien and Mushynski, 1998). A fourth neurofilament subunit was identified in the CNS, termed a-internexin, with peripherin is located in the PNS (Yuan et al, 2006; Beaulieu et al, 1999). Neurofilaments were identified in the 70s to function in increasing the diameter of neuronal axons, thereby facilitating electrical conduction and neuronal signalling (Friede and Samorajki, 1970), and are the most abundant cytoskeletal molecules in myelinated neurons. NEFL-H and NEFL-M are functionally redundant in the absence of NEFL, failing to form filaments; however, NEFL has been shown to function in an isolated fashion (Carter et al, 1998). The molecular structure of NEFL is comprised of a highly conserved 310 amino acid rod domain that allows interaction and co-assembly with other neurofilament subunits, and a microtubule polymerization inhibitory domain, which regulates the density of microtubules in the axon (Bocquet al, 2009).
NEFL Transport and Processing
NEFL is synthesized in the cell body of the neuron and is transported along to axon to reach its functional site. Many studies have investigated the molecular mechanisms governing this transport, with more recent evidence describing a ‘stop-and go’ model of neurofilament transport, suggesting that axons contain a single kinetic population of neurofilaments that can move in a bi-directional manner, by microtubule motor proteins, such as kinesin and dynein (Yuan et al, 2015; Li et al, 2012; Prahlad et al, 2000). NEFL is also transported back to the cell body in order to be degraded (Hoffman and Lasek, 1985). An important function of the rod domain of NEFL is to act as a binding site of motor proteins, such as myosin. Interactions between NEFL and motor proteins plays a crucial role in the topography and cellular localization of organelles, including the endoplasmic reticulum, endosomes and synaptic vesicles in the axon, with the deletion of either myosin or NEFL leading to the downregulation of these cellular organelles in the axon (Rao et al, 2011).
NEFL underdoes several post-translational modifications, such as phosphorylation, glycosylation, oxidation and ubiquitination (Perrot et al, 2008). The head domains of neurofilaments are glycosylated and phosphorylated, with phosphorylation of NEFL inhibiting neurofilament assembly (Yates et al, 2009; Hisinaga et al, 1990). This is proposed to be a self-regulating mechanism, as NEFL phosphorylation is upregulated following NEFL subunit synthesis (Sihag and Nixon, 1991). Phosphorylation of NEFL is mediated by protein kinase A and C (PKA and PKC). Transport along the axon requires dephosphorylation of NEFL, which is mediated by protein phosphatase 2A (PP2A) (Saito et al, 1995). Dysregulated phosphorylation of NEFL has been implicated in neurodegenerative disease (Dale and Garcia, 2012).
NEFL and Neuropathologies : A Role for NEFL as a Prognostic Biomarker
Disturbances in the metabolism and cellular localization of NEFL, as well as nefl mutations were shown to contribute to the pathogenesis of neurodegenerative diseases, such as Alzheimer’s disease and Charcot-Marie Tooth disease (Jordanova et al, 2003; Grierson et al, 2001). More recently, a Neuronal Intermediate Filament Inclusion disease has been described, which presents clinically similar to frontotemporal dementia (Bigio et al, 2003). NEFL can be secreted following neuronal damage, and increased levels of NEFL have been reported in several neuropathologies (Zetterberg, 2016; Perrot et al, 2008). NEFL is an important diagnostic marker for several neurodegenerative diseases, such as Amyotrophic Lateral Sclerosis (ALS) (Rosengren et al, 1996), Multiple Sclerosis (MS) (Tuenissen et al, 2009), and recently also described in Huntington’s disease (HD) (Niemela et al, 2017). In addition to the role of NEFL in neurodegenerative disease, elevated NEFL is reported in traumatic brain injury (TBI), stroke, and in HIV-associated dementia (Shahim et al, 2016; Gisslen et al, 2015). A recent study determined that NEFL is released from damage neurons, in response to accumulated protein inclusions, and effectively leaks out of the CSF and into the blood (Baciaglu et al, 2016). This is the first study to correlate the levels if NEFL in the CSF and the blood of neurodegenerative patients, and has major implications in the predictive biomarker field and also has potential to monitor therapeutic treatments in the clinic as it is easily accessible in the blood.
Figure 1: NEFL involvement in neurodegenerative disease. Accumulation of NEFL has been reported in multiple neurodegenerative pathologies, such as Alzheimer’s disease, Amyotrophic Lateral Sclerosis, and Huntington’s disease. Dysregulated post-translational modifications of NEFL and mutations in the nefl gene are implicated to lead to imbalanced NEFL levels in the axon. NEFL can be released from damaged neurons into the CSF. Recently, elevated levels of NEFL in the blood were demonstrated to reflect that of the CSF, implicating the promising potential of NEFL quantification as a biomarker for neuropathologies. Neuron outline sketch courtesy of Pixabay.
Check out our NEFL ELISA Kit
References
- Bacioglu M, Maia LF, Preische O, Schelle J, Apel A, Kaeser SA, Schweighauser M, Eninger T, Lambert M, Pilotto A, Shimshek DR, Neumann U, Kahle PJ, Staufenbiel M, Neumann M, Maetzler W, Kuhle J, Jucker M. Neurofilament Light Chain in Blood and CSF as Marker of Disease Progression in Mouse Models and in Neurodegenerative Diseases. Neuron. 2016. 91(1):56-66.
- Beaulieu JM, Robertson J, Julien JP. Interactions between peripherin and neurofilaments in cultured cells: disruption of peripherinassembly by the NF-M and NF-H subunits. Biochem Cell Biol. 1999;77(1):41-5.
- Bigio EH, Lipton AM, White CL 3rd, Dickson DW, Hirano A. Frontotemporal and motor neurone degeneration with neurofilament inclusion bodies: additionalevidence for overlap between FTD and ALS. Neuropathol Appl Neurobiol. 2003. 29(3):239-53.
- Bocquet A, Berges R, Frank R, Robert P, Peterson AC, Eyer J. Neurofilaments bind tubulin and modulate its polymerization. J Neurosci. 2009. 29(35):11043-54.
- Cardone B, Roots BI. Comparative immunohistochemical study of glial filament proteins (glial fibrillary acidic protein and vimentin) in goldfish, octopus, and snail. Glia. 1990. 3(3):180-92.
- Carter J, Gragerov A, Konvicka K, Elder G, Weinstein H, Lazzarini RA. Neurofilament (NF) assembly; divergent characteristics of human and rodent NF-L subunits. J Biol Chem. 1998. 273:5101–5108
- Dale JM, Garcia ML. Neurofilament Phosphorylation during Development and Disease: Which Came First, the Phosphorylation or the Accumulation? J Amino Acids. 2012. 382107.
- Friede RL, Samorajski T. Axon caliber related to neurofilaments and microtubules in sciatic nerve fibers of rats and mice. Anat Rec. 1970. 167(4):379-87.
- Gaiottino J, Norgren N, Dobson R, Topping J, Nissim A, Malaspina A, Bestwick JP, Monsch AU, Regeniter A, Lindberg RL, Kappos L, Leppert D, Petzold A, Giovannoni G, Kuhle J. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One. 2013. 8(9):e75091.
- Gisslén M, Price RW, Andreasson U, Norgren N, Nilsson S, Hagberg L, Fuchs D, Spudich S, Blennow K, Zetterberg H. Plasma Concentration of the Neurofilament Light Protein (NFL) is a Biomarker of CNS Injury in HIV Infection: A Cross-Sectional Study. EBioMedicine. 2015. 3:135-140.
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026