Narnatumab: Unveiling the Role of Anti-CD47 in Cancer Research
Quick Facts About Narnatumab
What is Narnatumab?
Narnatumab is a monoclonal antibody targeting CD47, a key immune checkpoint that helps cancer cells evade destruction by the immune system.
What is the mechanism of action for Narnatumab?
Narnatumab blocks CD47, a "don't eat me" signal used by cancer cells to avoid phagocytosis. This enhances macrophage-mediated immune responses against tumors.
What are the clinical applications of Narnatumab?
Narnatumab is being investigated for its potential in treating hematologic malignancies and solid tumors, with ongoing research exploring its efficacy in combination therapies.
1.) Understanding Narnatumab
Narnatumab is a promising therapeutic agent in the realm of immuno-oncology, particularly due to its ability to target CD47, an immune checkpoint protein that plays a critical role in immune evasion by cancer cells. The protein CD47 is often overexpressed in various cancer types, contributing to tumor progression by allowing cancer cells to escape detection by the immune system. This overexpression is frequently associated with poor prognosis in patients, making CD47 a key target for novel therapies. By blocking CD47, Narnatumab aims to enhance the body’s natural immune response, particularly through macrophage-mediated phagocytosis, to more effectively eliminate tumor cells. In doing so, Narnatumab helps reverse the tumor’s ability to evade immune surveillance, a fundamental challenge in cancer immunotherapy. As immunotherapy continues to evolve, Narnatumab is part of a rapidly growing class of anti-CD47 therapies under investigation in clinical trials. This class of therapies has generated significant interest in oncology research, given their potential to enhance immune system activity and improve patient outcomes.
Researchers are especially focused on evaluating Narnatumab’s effectiveness when combined with other therapeutic modalities, such as immune checkpoint inhibitors or traditional chemotherapy agents. These combination therapies may be particularly effective in cancers that are resistant to current treatment options. Given the growing body of preclinical evidence, Narnatumab holds promise as a valuable tool in the fight against various cancers, particularly hematologic malignancies and solid tumors. Ongoing research will determine its role in advancing cancer treatment and improving patient survival rates.
2.) Mechanism of Action of Narnatumab
The primary mechanism of Narnatumab is its ability to inhibit CD47, a cell surface protein that plays a crucial role in immune evasion by cancer cells. CD47 interacts with signal regulatory protein alpha (SIRPα) on macrophages, delivering a “don’t eat me” signal that protects tumor cells from being engulfed and destroyed by the immune system. By blocking this interaction, Narnatumab removes the protective signal, allowing the immune system, particularly macrophages, to recognize and phagocytize cancerous cells. This enhances the body’s natural defense mechanisms and enables it to target and eliminate malignant cells more effectively.
The inhibition of CD47 is significant because it not only blocks tumor immune evasion but also potentially improves the immune system’s ability to recognize tumor-associated antigens. Preclinical studies have suggested that Narnatumab can increase the activation of antigen-presenting cells, which further enhances the immune response and promotes broader immune activation. This dual mechanism of immune system activation makes Narnatumab a promising candidate for combination therapies with other immunotherapeutic agents, such as immune checkpoint inhibitors like anti-PD-1 or anti-CTLA-4 antibodies. By combining Narnatumab with other immunotherapies or even chemotherapy, researchers hope to achieve a more robust and sustained anti-tumor effect, particularly in cancers that have been resistant to monotherapy treatments. Ongoing studies are focused on understanding the optimal dosing strategies and evaluating the synergistic effects of Narnatumab when paired with other cancer treatments to improve patient outcomes and survival.
3.) Clinical Applications of Narnatumab
Narnatumab is currently being evaluated in various therapeutic areas, focusing on both hematologic malignancies and solid tumors. One of the primary areas of research involves its use in treating hematologic cancers, such as leukemia and lymphoma. These cancers are often characterized by high CD47 expression, which plays a key role in immune evasion and tumor progression. Preclinical studies have shown promising results, indicating that Narnatumab’s inhibition of CD47 could be particularly effective in treating these malignancies by enhancing macrophage-mediated phagocytosis and promoting a more robust immune response. In addition to hematologic cancers, Narnatumab is being investigated in the context of solid tumors, including breast, lung, and colorectal cancers, where immune evasion remains a significant challenge.
Many solid tumors overexpress CD47, allowing them to escape immune detection and continue growing unchecked. Narnatumab’s ability to block CD47 may help overcome this challenge and improve the effectiveness of treatment in solid tumors. Ongoing studies are also exploring the potential of combining Narnatumab with other cancer therapies, such as chemotherapy or immune checkpoint inhibitors. Early research suggests that this combination approach could lead to enhanced anti-tumor activity and better patient outcomes. While clinical trials are still in the early stages, Narnatumab is emerging as a promising candidate in the field of cancer immunotherapy. As research continues to evolve, investigators are focused on determining the optimal dosing strategies, patient populations that will benefit most from treatment, and the potential for combining Narnatumab with other therapeutic modalities to improve efficacy and survival rates.
4.) Exploring Biosimilars for Narnatumab
What is a Biosimilar?
A biosimilar is a biologic product highly similar to an existing reference biologic, with no clinically meaningful differences in terms of safety, purity, and potency. Biosimilars provide an essential tool for research and drug development, offering cost-effective alternatives for experimental studies.
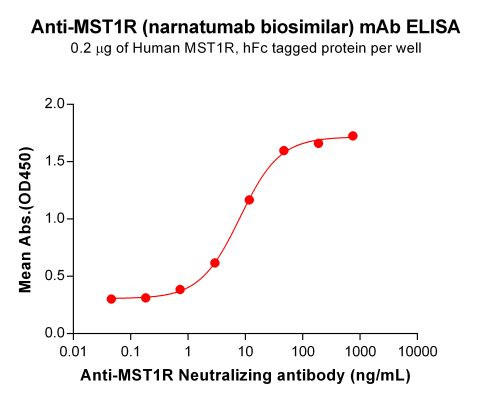
| Narnatumab (Anti-MST1R) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | MST1R |
| Reactivity: | Human |
How Narnatumab Biosimilar Compares to Narnatumab
Biosimilars of Narnatumab are designed to replicate its structure and function, allowing researchers to study its effects without the high costs associated with proprietary biologics. While biosimilars cannot be used interchangeably in clinical settings without regulatory approval, they serve as valuable research tools for preclinical studies and drug development.
Advancing Research on Narnatumab
Biosimilars provide a pathway for expanding research into CD47-targeting therapies. By making Narnatumab’s mechanism more accessible for investigation, biosimilars enable:
- Expanded Preclinical Research: Facilitating new insights into CD47 blockade.
- Combination Therapy Studies: Evaluating how CD47 inhibition interacts with other immunotherapies.
- Drug Development Efforts: Supporting innovation in novel cancer treatment strategies.
Research Use Only Disclaimer:
Narnatumab biosimilars are intended for research purposes only and are not approved for clinical use.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By David Lee, PhD
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



