Murlentamab: Revolutionizing Cancer Research with Targeted Therapy
Quick Facts About Murlentamab
What is Murlentamab?
Murlentamab is a novel monoclonal antibody designed to target CD47, a "don’t eat me" signal that helps cancer cells evade the immune system.
What is the mechanism of action for Murlentamab?
Murlentamab binds to CD47 on cancer cells, blocking its interaction with SIRPα on macrophages. This promotes phagocytosis, enabling the immune system to eliminate cancer cells more effectively.
What are the clinical applications of Murlentamab?
Murlentamab is primarily being explored for treating solid tumors and hematological malignancies. Emerging research suggests its potential in combination therapies for enhanced efficacy.
1.) Understanding Murlentamab
Murlentamab is a groundbreaking agent in the field of immuno-oncology, specifically designed to target CD47, a protein frequently overexpressed on the surface of cancer cells. CD47 acts as a "don't eat me" signal to the immune system, particularly macrophages, enabling cancer cells to evade immune detection and destruction. By inhibiting CD47, Murlentamab disrupts this mechanism, effectively exposing cancer cells to immune surveillance and promoting their elimination. This targeted approach has gained considerable attention due to its ability to selectively enhance anti-tumor immune responses while minimizing the risk of off-target effects that are common in other immunotherapies.
One of the most compelling aspects of Murlentamab is its versatility in combination therapies. When used alongside immune checkpoint inhibitors, such as anti-PD-1 or anti-CTLA-4 therapies, Murlentamab enhances T-cell activation and amplifies the immune response against tumors. Additionally, its integration with traditional chemotherapeutic agents has shown promise, as chemotherapy-induced immunogenic cell death may further augment the efficacy of CD47 inhibition. This synergistic interaction not only enhances therapeutic efficacy but also provides a broader treatment window for patients with aggressive or resistant cancers.
Moreover, preclinical and clinical trials suggest that Murlentamab exhibits a favorable safety profile, addressing concerns of widespread immune activation or hematological toxicities. This balance of efficacy and safety underscores its potential to redefine cancer treatment paradigms. As research and development progress, Murlentamab continues to highlight the evolving landscape of precision medicine, offering hope for improved outcomes in cancer care through its innovative mechanism and adaptability in multimodal treatment strategies.
2.) Mechanism of Action of Murlentamab
Murlentamab’s mechanism of action is rooted in its ability to neutralize the immunosuppressive effects of CD47, a protein widely recognized as a “do not eat me” signal. CD47 interacts with signal-regulatory protein alpha (SIRPα) receptors on macrophages, effectively inhibiting their ability to phagocytose cells. Cancer cells exploit this pathway by overexpressing CD47 on their surface, cloaking themselves from the immune system and evading destruction. This makes CD47 a critical target for therapeutic intervention in oncology.
Murlentamab works by binding specifically to CD47, blocking its interaction with SIRPα and disrupting this protective signal. This blockade reactivates macrophages, enabling them to recognize, engulf, and destroy malignant cells through phagocytosis. The process of tumor cell destruction also releases tumor-associated antigens into the microenvironment, which are subsequently presented to T cells. This antigen presentation enhances the activation of adaptive immune responses, driving a broader and more sustained attack on the tumor. By simultaneously engaging innate and adaptive immunity, Murlentamab facilitates a multifaceted immune assault on cancer.
One of the key advantages of Murlentamab is its specificity for CD47, which minimizes the risk of off-target effects and systemic toxicities often associated with non-selective therapies. Unlike earlier CD47 inhibitors that risked damage to normal cells, particularly red blood cells, Murlentamab’s precision design spares healthy tissues, offering a safer therapeutic profile. This precision addresses a significant challenge in cancer immunotherapy, ensuring effective tumor clearance while reducing adverse effects. Consequently, Murlentamab represents a highly promising and innovative strategy in cancer treatment.
3.) Clinical Applications of Murlentamab
Murlentamab has demonstrated considerable potential across a spectrum of cancer types, leveraging its ability to target CD47 and disrupt cancer’s evasion of immune surveillance. Its clinical applications span both hematological malignancies and solid tumors, making it a versatile therapeutic agent in oncology.
In hematological cancers such as non-Hodgkin lymphoma and acute myeloid leukemia, Murlentamab’s capacity to enhance immune-mediated clearance has shown significant promise. By reactivating macrophages to target malignant cells, it addresses a critical need for therapies capable of improving outcomes in these often-aggressive diseases. Preclinical and early clinical studies have highlighted its ability to induce durable responses, positioning it as a valuable addition to the treatment landscape for blood cancers.
In solid tumors, emerging evidence underscores its efficacy in cancers such as colorectal, ovarian, and breast cancer. Murlentamab is particularly effective when integrated into combination therapies. When paired with immune checkpoint inhibitors like anti-PD-1 or anti-CTLA-4 antibodies, it amplifies antitumor immune responses by simultaneously engaging both innate and adaptive immune systems. Similarly, combining Murlentamab with chemotherapy or radiation therapy has shown potential in overcoming tumor resistance mechanisms, enhancing overall therapeutic efficacy.
Looking to the future, ongoing research is focused on refining its clinical applications. Studies are optimizing dosing regimens to maximize effectiveness while minimizing toxicity. Efforts are also underway to explore novel delivery mechanisms, such as nanoparticle-based systems, to improve targeting and bioavailability. Additionally, researchers are expanding its applications to other cancer subtypes, paving the way for more personalized treatment strategies and advancing the frontier of cancer immunotherapy.
4.) Exploring Biosimilars for Murlentamab
What is a Biosimilar?
Biosimilars are biologic medical products that are highly similar to an already approved reference product, with no clinically meaningful differences in safety, purity, or potency. They play a crucial role in advancing research by providing cost-effective alternatives.
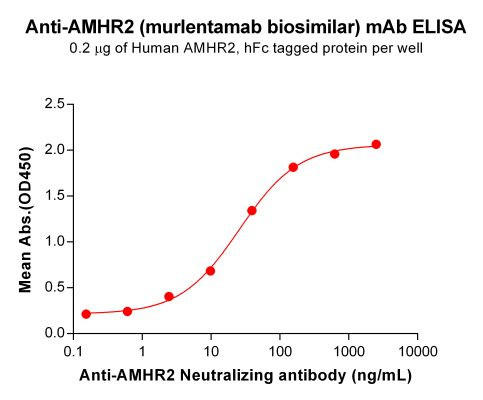
| Murlentamab (Anti-AMHR2) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | AMHR2 |
| Reactivity: | Human |
How Murlentamab Biosimilar Compares to Murlentamab
Our Murlentamab biosimilar offers researchers a valuable tool for exploring immuno-oncology pathways. While maintaining the structural and functional integrity of the original drug, the biosimilar is designed for research use only, ensuring accessibility for scientific investigations.
Benefits of the Biosimilar in Research
Cost-Effectiveness: Provides an affordable alternative for preclinical and exploratory studies.
Accessibility: Facilitates broader access to advanced research tools.
Reliability: Ensures consistent performance for reproducible results.Research Use Only Disclaimer:
The Murlentamab biosimilar is intended exclusively for research purposes and is not approved for clinical use. This distinction ensures compliance with regulatory standards while advancing scientific discovery.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Miren Ruiz de Eguilaz, PhD
Miren Ruiz de Eguilaz, PhD, has an extensive academic background, earning a BSc in Biology from UPV/EHU, an MSc in Biotechnology from the University of Oviedo, and a PhD in Chemistry from Dublin City University (DCU). Miren’s expertise lies in biosensor technology and bacterial diagnostics. She currently serves as a Product Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Ivuxolimab: Redefining Cancer Immunotherapy and Research
Quick Facts About IvuxolimabWhat is ivuxolimab?Ivuxolimab is a monoclonal antibody tar …1st Feb 2025


