Monalizumab: A Breakthrough in Cancer Immunotherapy Targeting NKG2A
Quick Facts About Monalizumab
What is Monalizumab?
Monalizumab is a first-in-class immune checkpoint inhibitor targeting NKG2A, developed to enhance anti-tumor immune response by blocking inhibitory signals in NK and T cells.
How Does Monalizumab Work?
Monalizumab blocks NKG2A, a receptor found on NK and CD8+ T cells, restoring their cytotoxic activity against tumor cells expressing HLA-E.
What Are the Clinical Applications of Monalizumab?
Monalizumab is being investigated for treating various cancers, including head and neck squamous cell carcinoma (HNSCC), lung cancer, and colorectal cancer, often in combination with checkpoint inhibitors like Durvalumab.
Is Monalizumab FDA-Approved?
Monalizumab is currently in clinical trials and has not yet received FDA approval.
1.) Understanding Monalizumab
Monalizumab is a novel monoclonal antibody developed to target NKG2A, a receptor present on natural killer (NK) cells and cytotoxic T lymphocytes (CTLs). NKG2A is part of the immunoregulatory pathway that suppresses the immune system's ability to recognize and destroy cancer cells. It does this by binding to its ligand, HLA-E, which is often overexpressed on tumor cells, particularly in the context of immune evasion. HLA-E is a major mechanism tumors use to escape detection and elimination by the immune system.
When NKG2A binds to HLA-E, it sends an inhibitory signal to NK cells and T-cells, reducing their ability to mount a strong immune response. This means that even though the immune system is capable of detecting and targeting cancer cells, the immune system is effectively silenced, allowing the tumor to proliferate unchecked. By inhibiting this interaction, Monalizumab prevents immune suppression, enhancing the ability of both NK cells and CD8+ T-cells to attack tumor cells.
Monalizumab is a promising therapeutic agent due to its distinct mechanism of action, which differentiates it from more commonly used immune checkpoint inhibitors like PD-1/PD-L1 blockers. The ability to modulate immune activity through this novel pathway makes Monalizumab a compelling candidate for combination therapy, especially when paired with other immunotherapies targeting PD-1/PD-L1. Preclinical and clinical studies suggest that Monalizumab can significantly increase immune cell activation, leading to more effective immune responses against a wide range of cancers, particularly those that are hard to treat with traditional therapies.
2.) Mechanism of Action of Monalizumab
The primary mechanism of action of Monalizumab is the inhibition of the NKG2A receptor, which is found on the surface of NK cells and cytotoxic T lymphocytes (CD8+ T-cells). NKG2A is an immune checkpoint receptor that serves as a negative regulator of immune function. In healthy immune surveillance, NK cells and CD8+ T-cells play a crucial role in recognizing and eliminating tumor cells. However, in the presence of NKG2A's interaction with its ligand HLA-E, these immune cells are suppressed, and their anti-tumor activity is diminished. This allows tumor cells expressing HLA-E to escape immune detection and growth.
Monalizumab works by binding directly to the NKG2A receptor and blocking its ability to interact with HLA-E. This blockade prevents the inhibitory signals from being transmitted to immune cells, effectively releasing the "brakes" on NK and T-cell activity. As a result, immune cells are able to resume their natural anti-tumor functions, which include direct cytotoxic activity against tumor cells and the production of cytokines that can further recruit and activate other immune cells to the tumor site.
The therapeutic potential of Monalizumab is amplified when combined with other immune checkpoint inhibitors such as PD-1/PD-L1 blockers, as this dual inhibition can produce synergistic effects. For example, when paired with Durvalumab, a PD-L1 inhibitor, Monalizumab has shown the ability to restore both innate (NK cell) and adaptive (CD8+ T-cell) immune responses, making it particularly effective in cancers that otherwise evade immune detection through HLA-E overexpression. This combination therapy strategy could significantly improve treatment outcomes for a variety of cancer types, especially those that are resistant to single-agent checkpoint inhibition.
3.) Clinical Applications of Monalizumab
Head and Neck Cancer Monalizumab has demonstrated potential in the treatment of recurrent or metastatic head and neck squamous cell carcinoma (HNSCC). In these cancers, the immune system is often thwarted by the overexpression of HLA-E on tumor cells, which leads to immune escape. When Monalizumab is used in combination with Cetuximab, an epidermal growth factor receptor (EGFR) inhibitor, it enhances anti-tumor immunity. Cetuximab targets EGFR on the surface of cancer cells, inhibiting their growth and promoting immune cell-mediated killing. By combining Monalizumab with Cetuximab, the immune system's ability to recognize and attack tumor cells is further boosted, making this combination therapy a promising approach in treating HNSCC, which has limited treatment options and poor prognosis in advanced stages.
Emerging studies are showing that Monalizumab’s ability to block NKG2A provides a distinct advantage over traditional monotherapies. The combination therapy seems to restore immune activity in a way that makes the tumor more susceptible to both immune-mediated destruction and the targeted action of Cetuximab. This suggests that Monalizumab could play a significant role in improving the clinical outcomes of patients with HNSCC, particularly those with advanced, treatment-resistant disease.
Lung Cancer Monalizumab is also being investigated in combination with checkpoint inhibitors for the treatment of non-small cell lung cancer (NSCLC), particularly in patients with high HLA-E expression. Lung cancer is notorious for its ability to suppress the immune response, and many patients with NSCLC experience poor outcomes with existing immunotherapies. The overexpression of HLA-E on tumor cells contributes to immune evasion by silencing NK and T-cell responses, making Monalizumab a promising therapeutic option for enhancing immune responses in these patients.
Clinical trials are exploring the combination of Monalizumab with PD-1 inhibitors like Pembrolizumab, with the goal of improving response rates in patients who express high levels of HLA-E. Preliminary data suggests that the addition of Monalizumab may help overcome resistance to PD-1/PD-L1 inhibitors by reactivating immune cells that are otherwise suppressed by HLA-E, potentially leading to more robust anti-tumor responses and improved survival outcomes for patients with NSCLC.
Colorectal Cancer Colorectal cancer, especially microsatellite-stable (MSS) colorectal cancer, remains a significant challenge in immunotherapy due to its limited response to existing treatments. However, the combination of Monalizumab with Durvalumab (a PD-L1 inhibitor) and Bevacizumab (an anti-VEGF agent) is showing promise in overcoming some of the barriers to treatment efficacy. In MSS colorectal cancer, where immunotherapy has historically been ineffective, the combined approach of targeting multiple immune checkpoint pathways could enhance immune recognition and destruction of tumor cells.
Monalizumab’s ability to restore NK and T-cell activation, coupled with the anti-angiogenic effects of Bevacizumab and the immune checkpoint blockade provided by Durvalumab, makes this combination a novel approach in treating MSS colorectal cancer. Clinical trials are currently underway to further explore the efficacy of this combination, and early results indicate that Monalizumab may be able to provide meaningful therapeutic benefit to patients with this difficult-to-treat cancer type. If successful, this treatment strategy could significantly expand the immunotherapy options for colorectal cancer patients, improving survival and quality of life.
4.) Exploring Biosimilars for Monalizumab
What is a Biosimilar?
Biosimilars are biologic medicines highly similar to already approved reference products. They offer researchers cost-effective alternatives for studying drug mechanisms, efficacy, and resistance pathways.
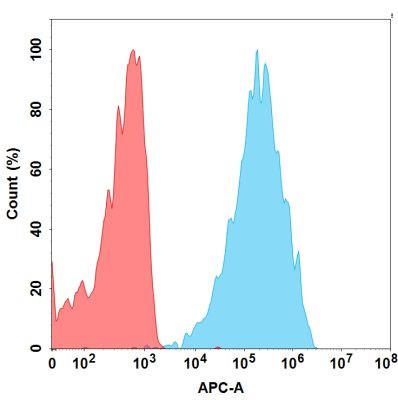
| Monalizumab (Anti-NKG2A) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | NKG2A |
| Reactivity: | Human |
How Monalizumab Biosimilar Compares to Monalizumab
- Similarity: Retains the same target specificity, binding affinity, and functional activity as the original Monalizumab.
- Differences: Used exclusively for research applications and not for clinical use or patient treatment.
- Advantages: Facilitates preclinical research, mechanism studies, and combination therapy exploration.
Advancing Research on Monalizumab
Monalizumab biosimilars provide a valuable tool for investigating:
- Alternative immune checkpoint blockade strategies.
- Combination immunotherapies.
- Mechanisms of tumor immune evasion.
Research Use Only Disclaimer:
Monalizumab biosimilar is for research purposes only and is not intended for human use.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Marina Alberto, PhD
Marina Alberto, PhD, holds a robust academic background in Biotechnology, earning her Bachelor’s Degree and PhD in Science and Technology from Quilmes National University. Her research spans cancer immunotherapy, glycan profiling, and vaccine development, including innovative projects on pediatric leukemia diagnosis and cancer-associated carbohydrate-mimetic vaccines. She currently serves as a Technical Support and Sales Specialist at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



