Mogamulizumab: Understanding Its Mechanism, Clinical Applications, and the Role of Biosimilars in Research
Quick Info Section: Key Facts About Mogamulizumab
What is Mogamulizumab?
What is the mechanism of action for Mogamulizumab?
Mogamulizumab works by targeting and blocking CCR4, a receptor on the surface of T-cells, which plays a crucial role in cancer cell migration and survival, thus helping to slow down the progression of certain malignancies.
What are the clinical applications of Mogamulizumab?
Mogamulizumab has been approved for the treatment of CTCL and ATLL, demonstrating efficacy in managing these cancers. Ongoing research explores its potential in other hematological malignancies and combination therapies.
1.) Understanding Mogamulizumab
Mogamulizumab is a humanized monoclonal antibody designed to target the chemokine receptor CCR4, which is primarily expressed on a subset of T-cells involved in the immune response. This receptor plays a crucial role in the migration of T-cells to tissues affected by malignancies. In certain cancers, including cutaneous T-cell lymphoma (CTCL) and adult T-cell leukemia/lymphoma (ATLL), CCR4 is overexpressed on malignant T-cells, which facilitates tumor cell migration, immune evasion, and disease progression. Mogamulizumab works by blocking the CCR4 receptor, preventing the malignant T-cells from reaching tumor sites where they could contribute to further proliferation.
This action not only interferes with the tumor cells’ ability to spread but also stimulates an immune response that targets the cancer cells. By enhancing immune cell activation through antibody-dependent cellular cytotoxicity (ADCC), Mogamulizumab further amplifies the body’s natural defenses, encouraging the immune system to recognize and destroy the cancer cells. This dual mechanism of blocking CCR4-mediated migration and promoting immune system activation makes Mogamulizumab a powerful and promising therapeutic agent in the treatment of hematologic cancers, particularly for patients with cancers that have proven resistant to other therapies.
Initially approved for treating CTCL and ATLL, Mogamulizumab’s clinical applications are expanding. Ongoing studies are evaluating its use in combination with other therapies, exploring its potential in a broader range of cancers. This novel mechanism of action represents a shift in how immunotherapies can be leveraged to treat difficult-to-manage cancers, offering new hope to patients with advanced disease.
2.) Mechanism of Action of Mogamulizumab
Mogamulizumab's mechanism of action revolves around its ability to bind specifically to CCR4, a receptor that plays a pivotal role in the trafficking and migration of T-cells to various tissues. CCR4 is expressed not only on healthy immune cells but also on malignant T-cells that contribute to the progression of certain cancers, such as CTCL and ATLL. Under normal conditions, CCR4 facilitates the migration of these T-cells to inflamed or malignant sites in the body, where they promote tumor growth and immune evasion. By targeting and blocking this receptor, Mogamulizumab prevents the cancerous T-cells from migrating to these areas, thereby impeding the spread of the tumor.
In addition to inhibiting CCR4-mediated migration, Mogamulizumab triggers immune activation through antibody-dependent cellular cytotoxicity (ADCC), a process where immune cells such as natural killer (NK) cells and macrophages recognize and kill antibody-coated tumor cells. This mechanism not only helps eliminate cancerous cells but also enhances the immune system’s ability to target other malignant cells that may have otherwise escaped detection. ADCC is an essential part of Mogamulizumab’s therapeutic profile, contributing to its ability to reduce tumor burden and improve patient outcomes.
The combination of blocking CCR4 and activating the immune system gives Mogamulizumab a dual action that is especially beneficial in cancers like CTCL and ATLL, where tumor progression is closely linked to T-cell trafficking. Researchers are increasingly interested in combining Mogamulizumab with other immunotherapies, such as immune checkpoint inhibitors, to further amplify its therapeutic effects and potentially extend its efficacy to other types of cancer. This ongoing exploration highlights the promising future of Mogamulizumab as a part of combination cancer therapies.
3.) Clinical Applications of Mogamulizumab
Mogamulizumab has already made a significant impact on the treatment landscape for hematologic malignancies, particularly in the management of cutaneous T-cell lymphoma (CTCL) and adult T-cell leukemia/lymphoma (ATLL). These rare and aggressive cancers often involve overexpression of CCR4 on malignant T-cells, making them difficult to treat with conventional therapies. Clinical trials have demonstrated that Mogamulizumab significantly improves overall survival and reduces tumor burden in patients with CTCL and ATLL, especially in those who have failed other treatment options. This has positioned Mogamulizumab as a breakthrough therapy for these difficult-to-treat cancers.
Further research is underway to assess Mogamulizumab's efficacy in combination with other immunotherapies, such as immune checkpoint inhibitors, to enhance therapeutic responses and overcome resistance mechanisms that are common in advanced cancers. The ability of Mogamulizumab to target CCR4 and activate immune responses makes it an attractive candidate for combination therapy in cancers where the immune system’s ability to recognize and destroy tumor cells is impaired. Studies are exploring the potential of using Mogamulizumab alongside chemotherapy, other monoclonal antibodies, and checkpoint inhibitors to improve treatment outcomes in patients with aggressive and treatment-resistant cancers.
Additionally, research is expanding into new areas, exploring the potential for Mogamulizumab in other hematologic malignancies and even solid tumors. The initial success in CTCL and ATLL has sparked further interest in investigating its broader applicability. A pivotal phase 3 trial has demonstrated its clinical benefits, and ongoing studies are examining its role in other cancers that involve similar mechanisms of immune evasion and T-cell dysfunction. As the research continues to evolve, Mogamulizumab’s role in immuno-oncology is expected to expand, offering more treatment options for patients facing challenging malignancies. This evolving body of evidence underscores the potential for Mogamulizumab to become a cornerstone of cancer immunotherapy.
4.) Advancing Research on Mogamulizumab with Biosimilars
What is a Biosimilar?
A biosimilar is a biologic medical product highly similar to an approved reference product. It is designed to match the reference drug in terms of structure, function, and therapeutic effects, but can be offered at a lower cost. In cancer research, biosimilars like Mogamulizumab Biosimilar (available for research use only) play a critical role in reducing the financial barriers for extensive testing, while still maintaining the scientific integrity of the studies.
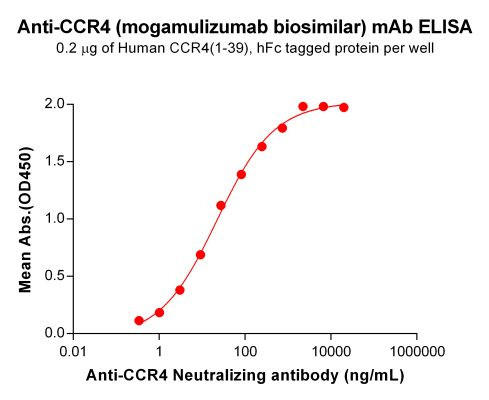
| Mogamulizumab (Anti-CCR4) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | CCR4 |
| Reactivity: | Human |
How Mogamulizumab Biosimilar Compares to Mogamulizumab
Biosimilars like Mogamulizumab Biosimilar (our research product) offer promising avenues for advancing the study of Mogamulizumab’s therapeutic potential. While the original Mogamulizumab is an effective treatment option, the biosimilar version offers advantages for research due to its cost-effectiveness and availability, providing researchers with a robust tool to deepen the understanding of this targeted therapy’s mechanisms.
Biosimilars are designed to be highly similar to their reference products, ensuring that they replicate the same therapeutic effects. These advantages are particularly important for the ongoing investigation into the optimal uses of Mogamulizumab in cancer therapies. By leveraging the biosimilar, researchers can explore various dosages, combinations, and long-term effects without the higher costs associated with the original product.
Benefits of Mogamulizumab Biosimilar in Research
The introduction of biosimilars into research environments holds significant benefits. It increases access to critical therapies for clinical trials, reduces research costs, and enhances the study of complex diseases like cancer. The use of Mogamulizumab Biosimilar allows for more detailed exploration of CCR4-targeting strategies, paving the way for new combinations and treatment regimens.
Researchers benefit from the ability to use a cost-effective version of the drug, ensuring that studies can include a larger number of participants and testing conditions. The biosimilar allows for a deeper understanding of long-term safety and efficacy in various therapeutic contexts.
Research Use Only Disclaimer:
It’s important to note that Mogamulizumab Biosimilar is intended for research use only. This allows researchers to further explore its potential without direct clinical applications, opening up a range of new possibilities for optimizing cancer therapies.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By David Lee, PhD
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



