Lorvotuzumab: Targeting CD56 in Cancer Research and Therapeutic Development
Quick Facts About Lorvotuzumab
What is Lorvotuzumab?
Lorvotuzumab is an antibody-drug conjugate (ADC) designed to target CD56, a surface antigen commonly overexpressed in neuroendocrine tumors and hematologic malignancies.
What is the mechanism of action of Lorvotuzumab?
Lorvotuzumab binds to CD56-positive cancer cells, delivering a cytotoxic payload (mertansine) to induce apoptosis and inhibit tumor growth.
What are the clinical applications of Lorvotuzumab?
Research on Lorvotuzumab has focused on treating small cell lung cancer (SCLC), multiple myeloma, and acute myeloid leukemia (AML). While some trials were discontinued, interest in its mechanism persists in oncology.
1.) Understanding Lorvotuzumab
Lorvotuzumab, also known as IMGN901, is an antibody-drug conjugate (ADC) developed by ImmunoGen, designed to target CD56-expressing cancers. CD56, a cell surface adhesion molecule, is overexpressed in various malignancies, particularly neuroendocrine tumors like small cell lung cancer (SCLC), neuroblastoma, and certain types of leukemia. The purpose of Lorvotuzumab is to improve treatment outcomes for these cancers by delivering a potent cytotoxic agent directly to the cancer cells, thereby minimizing damage to healthy tissues.
The mechanism behind Lorvotuzumab's potential lies in its ability to specifically bind to CD56, a receptor found on the surface of tumor cells, which enables the selective targeting of cancerous cells while sparing normal, healthy cells. Once the ADC binds to CD56-expressing cells, it is internalized, and the potent chemotherapy drug, DM1, is released inside the cell. This targeted delivery helps to overcome the challenges of traditional chemotherapy, which indiscriminately damages both cancerous and healthy cells. This approach promises to enhance therapeutic efficacy while reducing the risk of side effects, a key challenge in cancer treatment.
However, despite promising preclinical findings, Lorvotuzumab's clinical trials have faced setbacks, particularly in terms of safety and toxicity concerns. These challenges have limited its widespread use in clinical settings. Despite these hurdles, the scientific community remains committed to exploring Lorvotuzumab’s potential for targeted cancer therapy. Ongoing research is focused on optimizing its design, improving its safety profile, and exploring its use in combination therapies with other cancer treatments. Its specificity for CD56-expressing tumors remains a compelling reason to continue investigating its therapeutic applications, particularly in rare or hard-to-treat cancers, where effective treatment options are often limited.
2.) Mechanism of Action of Lorvotuzumab
Lorvotuzumab functions as an antibody-drug conjugate (ADC), a therapeutic approach designed to deliver powerful cytotoxic agents directly to cancer cells, minimizing collateral damage to healthy tissues. It consists of a monoclonal antibody that specifically binds to CD56, a surface protein that is overexpressed on a range of tumors, including small cell lung cancer (SCLC), neuroblastoma, and certain leukemias. This selective binding to CD56 is a critical feature of Lorvotuzumab, as it ensures that the cytotoxic drug it carries—DM1 (mertansine), a microtubule-disrupting agent—only affects the targeted tumor cells.
Once Lorvotuzumab binds to CD56 on the surface of a cancer cell, the ADC is internalized into the cell via receptor-mediated endocytosis. Inside the cell, the linker that connects the monoclonal antibody to DM1 breaks down, releasing the cytotoxic drug. DM1 then interferes with the microtubule network within the cancer cell, halting cell division and triggering apoptosis (programmed cell death). This process leads to the regression of the tumor, as the targeted cells are unable to divide and proliferate. By delivering DM1 specifically to cancer cells, Lorvotuzumab offers the potential for highly targeted therapy with reduced side effects compared to traditional chemotherapy.
While the concept of ADCs holds great promise, clinical trials for Lorvotuzumab have faced challenges, particularly in terms of safety and toxicity. However, the underlying mechanism of action—targeted delivery of a potent chemotherapeutic agent—remains an important focus for future drug development, especially in the context of combination therapies to enhance efficacy while minimizing adverse effects.
3.) Clinical Applications of Lorvotuzumab
Lorvotuzumab has been investigated in several clinical settings, primarily for the treatment of cancers expressing CD56, such as small cell lung cancer (SCLC), neuroblastoma, and hematologic malignancies like multiple myeloma and acute myeloid leukemia (AML). Preclinical studies and early-phase trials showed that Lorvotuzumab, when used as a monotherapy or in combination with other treatments, could effectively target and eliminate CD56-positive tumor cells. However, while these studies demonstrated promising anti-tumor effects, several clinical trials faced significant safety concerns, particularly related to the drug's toxicity. This has led to discontinuation or pause in the clinical development of Lorvotuzumab in certain indications, such as SCLC, where toxicity levels hindered further progression.
One of the most notable areas of investigation has been in small cell lung cancer (SCLC), a highly aggressive form of lung cancer that often presents with poor prognosis and limited treatment options. Despite initial signals of efficacy, clinical trials were discontinued due to adverse events. In other cancers, like neuroblastoma, which primarily affects children, Lorvotuzumab is being explored for its potential to treat rare pediatric tumors that express CD56. Research continues in these areas to assess its viability as a treatment for neuroblastoma and other CD56-positive cancers.
Moreover, ongoing efforts focus on improving the safety and efficacy of Lorvotuzumab, especially through optimizing ADC technology and exploring new derivatives of the drug. These efforts aim to reduce the toxicity associated with the therapy while enhancing its anti-tumor properties. Combining Lorvotuzumab with other cancer treatments, such as checkpoint inhibitors or chemotherapy, is also being considered to overcome its limitations and provide more effective treatment options for patients with hard-to-treat cancers. The refinement of ADCs and targeted therapies remains a critical area of cancer research, with Lorvotuzumab serving as an important example of the potential of this approach.
4.) Exploring Biosimilars for Lorvotuzumab
Biosimilars provide cost-effective alternatives for advancing oncology research. A biosimilar to Lorvotuzumab serves as a valuable tool for preclinical studies and drug development.
What is a Biosimilar?
A biosimilar is a biologic product highly similar to an approved reference biologic, maintaining comparable efficacy, safety, and immunogenicity. Unlike generics, biosimilars undergo rigorous evaluation to confirm similarity.
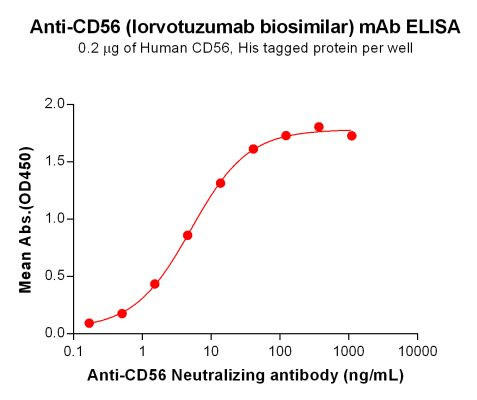
| Lorvotuzumab (Anti-CD56) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | CD56 |
| Reactivity: | Human |
How Lorvotuzumab Biosimilar Compares to Lorvotuzumab
A biosimilar to Lorvotuzumab mirrors the original’s CD56-binding properties, making it a crucial asset for:
- Investigating novel ADC formulations.
- Studying tumor microenvironment interactions.
- Developing combination therapies in oncology research.
Advancing Research on Lorvotuzumab
The availability of a Lorvotuzumab biosimilar facilitates ongoing discoveries in cancer treatment. Researchers leverage biosimilars to:
- Conduct preclinical trials on CD56-targeted therapies.
- Enhance ADC conjugate stability and safety.
- Investigate resistance mechanisms in relapsed cancers.
Research Use Only Disclaimer:
Lorvotuzumab biosimilars are intended for investigative purposes and not for direct clinical applications.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By David Lee, PhD
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Erenumab: Transforming Migraine Prevention Through CGRP Receptor Inhibition
Quick Facts About ErenumabWhat is Erenumab?Erenumab is a fully human monoclonal antibo …1st Apr 2025


