Labetuzumab: Revolutionizing Cancer Research with Targeted Therapies
Quick Facts About Labetuzumab
What is Labetuzumab?
Labetuzumab is a monoclonal antibody targeting CEACAM5, a protein overexpressed in various cancers, primarily colorectal carcinoma.
How does Labetuzumab work?
By binding to CEACAM5, Labetuzumab delivers cytotoxic agents directly to cancer cells, minimizing damage to healthy tissue.
What are its clinical applications?
Labetuzumab is primarily used in cancer treatment research, including colorectal cancer and other CEACAM5-positive malignancies.
Is Labetuzumab discontinued?
Some variants, like Labetuzumab govitecan, have been discontinued, but they remain vital in understanding targeted drug design.
1.) Understanding Labetuzumab
Labetuzumab represents a remarkable advance in the field of targeted cancer therapies. It is a monoclonal antibody specifically designed to recognize and bind to CEACAM5 (carcinoembryonic antigen-related cell adhesion molecule 5), a glycoprotein frequently overexpressed in various cancers, most notably colorectal cancer. This precise targeting capability enables Labetuzumab to selectively bind to cancer cells while sparing healthy tissues, reducing the adverse side effects typically associated with conventional chemotherapy. The unique mechanism of this antibody underscores its potential in improving patient outcomes by combining efficacy with a better safety profile.
Recent studies have highlighted Labetuzumab’s potential as an effective delivery platform for cytotoxic agents. By conjugating it with potent chemotherapeutics such as SN-38, the active metabolite of irinotecan, Labetuzumab ensures precise drug delivery to tumor sites, enhancing therapeutic outcomes. Formulations like Labetuzumab govitecan aimed to exploit this approach, although some have faced discontinuation due to challenges in clinical trials. However, these efforts provided valuable insights into the development and refinement of antibody-drug conjugates (ADCs). Researchers are actively exploring the use of Labetuzumab in innovative combination therapies to address challenges such as tumor heterogeneity and resistance mechanisms. As a result, Labetuzumab’s role in advancing personalized cancer treatments continues to be of great interest, cementing its importance in oncology research.
2.) Mechanism of Action of Labetuzumab
Labetuzumab functions as a highly selective monoclonal antibody that targets CEACAM5, a glycoprotein prominently expressed on cancer cell surfaces. Its mode of action includes several key mechanisms that make it a powerful tool in oncology. First, through antibody-dependent cellular cytotoxicity (ADCC), Labetuzumab recruits immune effector cells, such as natural killer (NK) cells, to recognize and destroy CEACAM5-expressing tumor cells. This immune-mediated response enhances the body’s ability to combat cancer.
When conjugated with cytotoxic agents like SN-38, Labetuzumab serves as a delivery vehicle, transporting the drug directly to the tumor site. This targeted approach minimizes systemic drug exposure, thereby reducing off-target toxicities while increasing drug concentration in the tumor microenvironment. Furthermore, Labetuzumab’s high affinity for CEACAM5 ensures that its binding remains highly specific, minimizing unintended interactions with healthy cells and improving its overall safety profile.
Advancements in ADC technology have further augmented Labetuzumab’s potential. Innovations in linker stability and optimization of drug-to-antibody ratios have enhanced its therapeutic efficacy. Stable linkers prevent premature drug release, while optimized ratios ensure an ideal balance between efficacy and tolerability. These advancements highlight Labetuzumab’s adaptability and pave the way for the development of next-generation ADCs, setting new standards for precision oncology.

3.) Clinical Applications of Labetuzumab
Labetuzumab holds significant promise across a range of cancer types, with its primary focus on colorectal cancer due to the frequent overexpression of CEACAM5 in these tumors. Clinical studies have demonstrated that Labetuzumab improves tumor control and extends progression-free survival in patients with CEACAM5-positive colorectal cancer. These findings highlight its potential to address unmet needs in managing this aggressive malignancy.
Beyond colorectal cancer, Labetuzumab is being investigated in other CEACAM5-positive cancers, such as lung and pancreatic cancers. Preliminary research indicates promising outcomes, with ongoing clinical trials aiming to explore its utility further in these and other malignancies. Its versatility as a targeted therapeutic opens doors to treating a broader range of cancers characterized by CEACAM5 overexpression.
Combination therapies represent another exciting frontier for Labetuzumab. By integrating it with immunotherapies such as checkpoint inhibitors, researchers aim to overcome tumor resistance mechanisms and amplify the immune system’s ability to eradicate cancer. This synergistic approach has shown potential in addressing the challenges posed by tumor heterogeneity and immune evasion. Despite the discontinuation of specific formulations like Labetuzumab govitecan, the foundational insights gained have significantly influenced the development of ADCs. Ongoing investigations into Labetuzumab’s applications continue to refine its role in precision oncology, ensuring its contributions remain integral to the future of cancer treatment.
4.) Exploring Biosimilars for Labetuzumab
Biosimilars of Labetuzumab are emerging as essential tools in cancer research. These products are designed to closely replicate the structure and function of the original antibody, offering researchers cost-effective alternatives for preclinical studies.
What is a Biosimilar?
A biosimilar is a biologic product that is highly similar to an already-approved reference product, with no clinically meaningful differences in safety or efficacy. In the context of Labetuzumab, biosimilars provide:
- Access to Advanced Research Tools: Facilitating studies in oncology without the high costs associated with original biologics.
- Consistency in Preclinical Testing: Ensuring reliable and reproducible results.
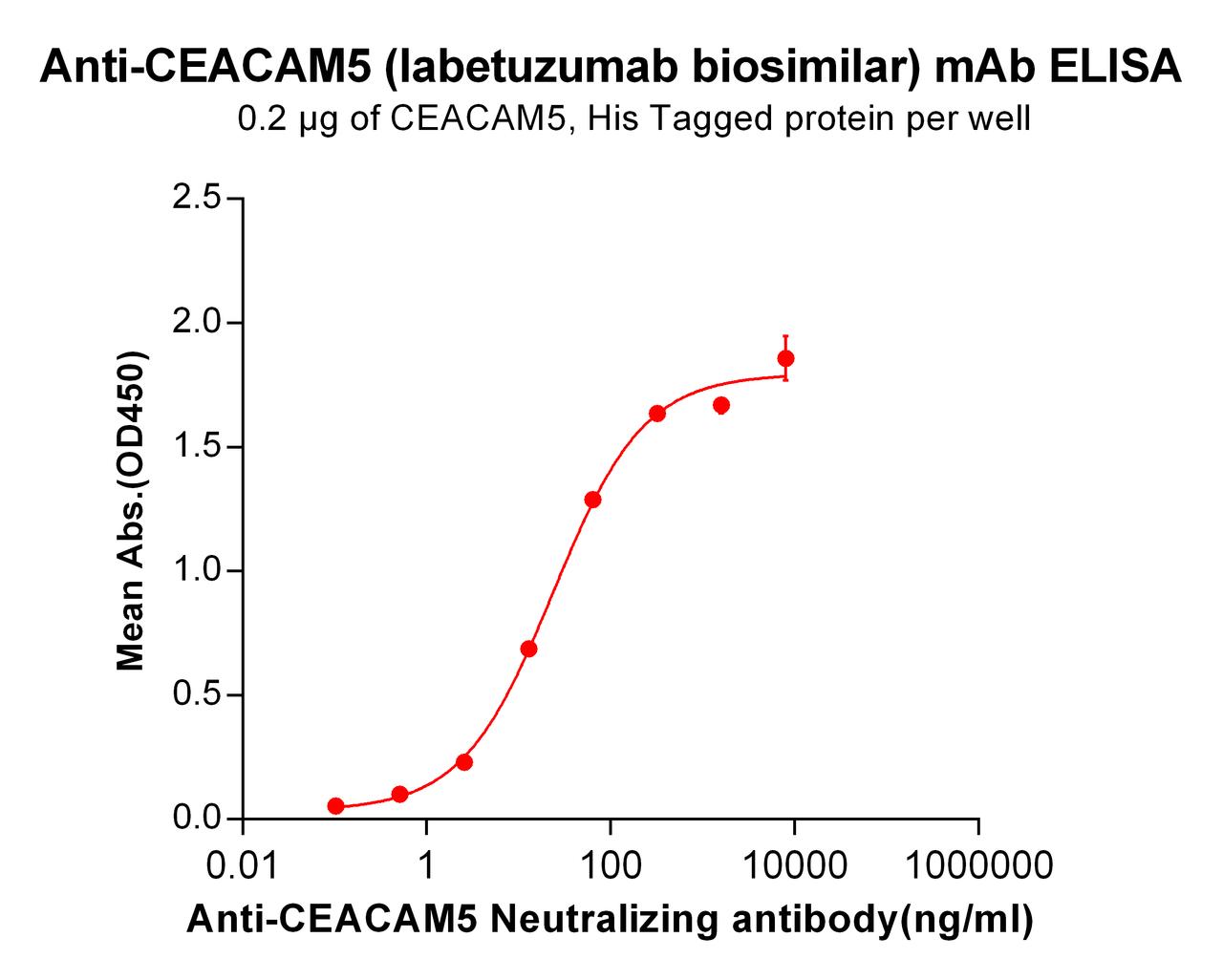
| Labetuzumab (Anti-CEACAM5) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | CEACAM5 |
| Reactivity: | Human |
How Labetuzumab Biosimilars Compare
- Similarities: Biosimilars retain the original drug’s specificity and efficacy in targeting CEACAM5.
- Differences: While biosimilars are not identical, minor variations in manufacturing processes do not affect their research utility.
Benefits of Labetuzumab Biosimilars
- Affordability: Lower costs enable wider access for academic and industrial researchers.
- Scalability: Increased availability supports large-scale studies and accelerates discovery.
- Ethical Research Practices: Reducing dependency on expensive biologics minimizes barriers to innovation.
Research Use Only Disclaimer:
Labetuzumab biosimilars are for research purposes only and are not approved for clinical use. Researchers must adhere to local regulations when utilizing these products.
Conclusion
Labetuzumab continues to influence the landscape of targeted cancer therapies. Its specificity for CEACAM5, combined with advancements in ADC technology, underscores its importance in oncology research. Biosimilars further democratize access to this groundbreaking antibody, enabling researchers to explore new therapeutic avenues efficiently and cost-effectively.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Miren Ruiz de Eguilaz, PhD
Miren Ruiz de Eguilaz, PhD, has an extensive academic background, earning a BSc in Biology from UPV/EHU, an MSc in Biotechnology from the University of Oviedo, and a PhD in Chemistry from Dublin City University (DCU). Miren’s expertise lies in biosensor technology and bacterial diagnostics. She currently serves as a Product Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025




