Ivuxolimab: Redefining Cancer Immunotherapy and Research
Quick Facts About Ivuxolimab
What is ivuxolimab?
Ivuxolimab is a monoclonal antibody targeting CD47, a "don't eat me" signal that cancer cells exploit to evade immune destruction.
How does ivuxolimab work?
By blocking CD47, ivuxolimab restores macrophage activity, enhancing the immune system’s ability to recognize and destroy cancer cells.
What are the clinical applications of ivuxolimab?
It is being explored in treating hematologic malignancies and solid tumors, with ongoing studies highlighting its potential in combination therapies.
Is ivuxolimab safe?
Early studies suggest a manageable safety profile, with most adverse events being mild to moderate and related to on-target effects.
1.) Understanding Ivuxolimab
Ivuxolimab represents a cutting-edge advancement in immuno-oncology by targeting CD47, a protein frequently overexpressed in various cancers as a mechanism of immune evasion. CD47 functions as a "don’t eat me" signal, binding to signal regulatory protein alpha (SIRPα) on macrophages and preventing the immune system from recognizing and eliminating cancer cells. This evasion strategy allows tumors to grow unchecked and resist immune-mediated destruction. Ivuxolimab disrupts this interaction, effectively removing the inhibitory signal and enabling macrophages to identify and phagocytose malignant cells, restoring immune surveillance against cancer.
The significance of ivuxolimab extends beyond its primary mechanism, representing a paradigm shift in cancer immunotherapy. By engaging both innate and adaptive immune responses, ivuxolimab enhances tumor clearance across various cancer types, including both primary and metastatic disease. Preclinical and clinical studies suggest that its efficacy is particularly notable when used in combination with other immune checkpoint inhibitors, such as anti-PD-1 and anti-CTLA-4 therapies, or with standard treatments like chemotherapy. Researchers are also investigating its application in hematologic malignancies like myelodysplastic syndromes (MDS) and lymphomas, as well as solid tumors such as ovarian, lung, and colorectal cancers.
Ivuxolimab’s development underscores the growing focus on targeting innate immune pathways in cancer treatment. By leveraging a novel immune-activating mechanism, it contributes to the expanding arsenal of precision oncology therapies. Its ability to enhance immune surveillance while maintaining a manageable toxicity profile makes ivuxolimab a promising candidate for improving survival outcomes and quality of life in patients with difficult-to-treat malignancies. As ongoing trials continue to evaluate its broader applications, ivuxolimab may emerge as a key component of next-generation cancer immunotherapy strategies.
2.) Mechanism of Action of Ivuxolimab
Ivuxolimab’s mechanism of action is centered on its ability to inhibit CD47, a cell surface protein that plays a crucial role in immune evasion. CD47 binds to signal regulatory protein alpha (SIRPα), a receptor present on macrophages, transmitting an inhibitory signal that prevents phagocytosis. This interaction enables cancer cells to evade immune destruction and continue proliferating unchecked. Ivuxolimab disrupts this pathway by blocking CD47, thereby reactivating macrophage-mediated phagocytosis and restoring the immune system’s ability to recognize and eliminate malignant cells.
Beyond its role in enhancing innate immunity, ivuxolimab also stimulates an adaptive immune response. As macrophages engulf and digest cancer cells, they release tumor-associated antigens, which are subsequently presented to T cells. This antigen presentation process initiates a broader immune response, leading to sustained tumor control through cytotoxic T-cell activation. This dual engagement of both innate and adaptive immunity positions ivuxolimab as a promising next-generation immunotherapy capable of generating durable anti-tumor responses. Additionally, its ability to promote immune memory may offer long-term protection against cancer recurrence.
Another critical advantage of ivuxolimab is its ability to selectively enhance immune function without causing widespread immune suppression. Unlike other immune checkpoint inhibitors that may lead to systemic inflammation or autoimmune complications, ivuxolimab’s targeted mechanism minimizes these risks. Preclinical studies and early clinical trials have demonstrated significant tumor regression, even in aggressive and treatment-resistant cancers, while maintaining a manageable safety profile. As research progresses, ivuxolimab is expected to be integrated into combination treatment strategies with existing immunotherapies, targeted therapies, and chemotherapy to further optimize its clinical benefits and broaden its applicability in oncology.
3.) Clinical Applications of Ivuxolimab
Ivuxolimab has demonstrated significant therapeutic potential across multiple cancer types, including both hematologic malignancies and solid tumors. Its ability to enhance immune-mediated tumor clearance has positioned it as a promising candidate for treating cancers that have historically been resistant to conventional therapies.
Hematologic Malignancies
Ivuxolimab has shown encouraging results in the treatment of hematologic cancers such as myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). These malignancies often exploit immune evasion mechanisms to resist standard treatments. Clinical studies suggest that combining ivuxolimab with hypomethylating agents like azacitidine can enhance therapeutic efficacy by targeting both malignant cells and the supportive tumor microenvironment. This combination strategy has demonstrated potential in improving patient outcomes by reducing tumor burden and enhancing immune clearance.
Solid Tumors
Ivuxolimab’s role in treating solid tumors is an area of active investigation. Preliminary clinical trials have reported promising responses in ovarian, colorectal, and breast cancers, particularly when ivuxolimab is combined with chemotherapy or immune checkpoint inhibitors. The synergy between these treatments helps overcome resistance mechanisms that often limit the effectiveness of standard therapies. By disrupting tumor immune evasion, ivuxolimab enhances the immune system’s ability to attack cancer cells more effectively.
Combination Therapies and Future Directions
Ongoing research is focused on exploring ivuxolimab in combination with anti-PD-1 or anti-PD-L1 therapies. By simultaneously targeting distinct immune pathways, these combination strategies amplify anti-tumor immunity, improving response rates and addressing tumor heterogeneity. Future studies are also evaluating ivuxolimab’s role in other cancers and chronic conditions characterized by immune escape mechanisms. Its potential inclusion in precision medicine frameworks underscores its adaptability and relevance in the evolving landscape of cancer treatment.
4.) Advancing Research on Ivuxolimab
What is a Biosimilar?
A biosimilar is a biologic medicine designed to be nearly identical to an already approved reference product in terms of safety, efficacy, and quality. Biosimilars offer cost-effective alternatives that broaden access to advanced therapies and drive innovation in biomedical research.
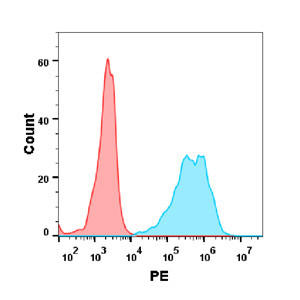
| Ivuxolimab (Anti-OX40) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | OX40 |
| Reactivity: | Human |
How Does Ivuxolimab Biosimilar Compare?
Our ivuxolimab biosimilar replicates the structure and function of the original, providing comparable efficacy and safety. However, it is specifically optimized for research use, enabling scientists to explore new therapeutic combinations and refine existing protocols.
Benefits of Ivuxolimab Biosimilar for Research
- Affordability: Lowers the cost of preclinical and exploratory studies, facilitating broader access to cutting-edge research tools.
- Availability: Increases the accessibility of biologics for academic and industrial research.
- Innovation: Supports the development of next-generation therapies by serving as a versatile tool in immuno-oncology research.
Note: The ivuxolimab biosimilar is intended for research use only and is not approved for clinical application.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Miren Ruiz de Eguilaz, PhD
Miren Ruiz de Eguilaz, PhD, has an extensive academic background, earning a BSc in Biology from UPV/EHU, an MSc in Biotechnology from the University of Oviedo, and a PhD in Chemistry from Dublin City University (DCU). Miren’s expertise lies in biosensor technology and bacterial diagnostics. She currently serves as a Product Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



