Indatuximab: Advancing CD138-Targeted Therapy in Cancer Research
What You Need to Know About Indatuximab
What is Indatuximab?
Indatuximab ravtansine is an experimental monoclonal antibody-drug conjugate (ADC) that targets CD138, a protein highly expressed on the surface of certain cancer cells, including multiple myeloma and some solid tumors.
What is the mechanism of action for Indatuximab?
Indatuximab ravtansine delivers a cytotoxic agent directly to cancer cells by binding to CD138, ensuring selective killing of malignant cells while sparing healthy tissues.
What are the clinical applications of Indatuximab?
Indatuximab ravtansine is being explored in clinical trials for its potential to treat relapsed and refractory multiple myeloma as well as renal cell carcinoma (RCC).
1.) Understanding Indatuximab
Indatuximab ravtansine, also known as BT062, is an advanced antibody-drug conjugate (ADC) that represents a significant innovation in cancer therapy. It is specifically designed to target CD138 (syndecan-1), a cell surface protein that is highly expressed in multiple myeloma and several other malignancies. CD138 plays a critical role in cell adhesion, migration, and signaling, processes that are often co-opted by cancer cells to promote growth and metastasis. This makes CD138 a valuable and strategic target for therapeutic intervention.
The mechanism of action of Indatuximab ravtansine combines the precision of monoclonal antibody targeting with the potent cytotoxicity of chemotherapy. The antibody component of Indatuximab selectively binds to CD138 on the surface of cancer cells, ensuring targeted delivery. Once bound, the ADC is internalized by the cancer cell, where the cytotoxic payload, a maytansinoid derivative (DM4), is released. This payload disrupts microtubule dynamics, leading to cell cycle arrest and subsequent apoptosis of the cancer cell.
By delivering its cytotoxic agent directly to cancer cells expressing CD138, Indatuximab ravtansine minimizes off-target effects and reduces systemic toxicity. Its targeted approach makes it particularly promising for treating multiple myeloma, where CD138 is consistently overexpressed, and ongoing research is exploring its potential in other malignancies that also exhibit high CD138 expression.
Prefer to Listen? Check out the Indatuximab Podcast Episode
2.) Mechanism of Action of Indatuximab
Indatuximab ravtansine is an antibody-drug conjugate (ADC) targeting CD138 (syndecan-1), a protein overexpressed on plasma cells and certain cancers, including multiple myeloma. The ADC consists of a monoclonal antibody, a cleavable linker, and DM4, a cytotoxic payload.
The mechanism begins with the ADC binding to CD138 on cancer cells, ensuring specificity. Upon binding, the complex is internalized via receptor-mediated endocytosis and transported to the lysosomal compartment. Within the acidic lysosomal environment, the linker is cleaved, releasing DM4 into the cytoplasm.
DM4, a maytansinoid derivative, disrupts microtubule assembly by binding to tubulin, leading to cell cycle arrest in the G2/M phase. This disruption activates apoptotic pathways, resulting in targeted cancer cell death.
Indatuximab ravtansine enhances therapeutic precision, reducing off-target effects and systemic toxicity compared to traditional chemotherapy. By combining targeted antibody delivery with the potent cytotoxicity of DM4, it offers a robust approach to treating CD138-positive malignancies, providing efficacy while minimizing harm to healthy tissues.

3.) Clinical Applications of Indatuximab
Indatuximab ravtansine, an antibody-drug conjugate (ADC), is under active investigation for its potential in treating various malignancies, particularly those characterized by high CD138 expression. Its design leverages the targeting of syndecan-1 (CD138), a surface marker prevalent in certain cancers, allowing precise delivery of its cytotoxic payload to tumor cells while minimizing off-target effects.
Relapsed and Refractory Multiple Myeloma:
Clinical trials have shown promising results for indatuximab ravtansine in relapsed and refractory multiple myeloma (RRMM). When combined with agents like pomalidomide and dexamethasone, it has demonstrated improved response rates and progression-free survival in patients who have exhausted other therapeutic options. This combination highlights its synergistic potential in overcoming resistance to standard therapies, offering renewed hope for heavily pretreated patients.
Renal Cell Carcinoma (RCC):
Emerging research is exploring the efficacy of indatuximab in renal cell carcinoma (RCC), a malignancy with limited targeted therapies. Its CD138-targeting mechanism may prove valuable in addressing CD138-expressing RCC tumors, expanding its applicability beyond hematological cancers.
Ongoing studies aim to optimize indatuximab’s safety profile, determine its most effective dosing strategies, and explore its utility in combination regimens. These efforts underscore the growing interest in ADCs as precision oncology tools capable of transforming cancer treatment paradigms.
4.) Advancing Research on Indatuximab
What is a Biosimilar?
A biosimilar is a biologic product that is highly similar to an already approved reference biologic, with no clinically meaningful differences in safety, purity, or potency. Biosimilars play a crucial role in expanding access to innovative therapies and enabling cost-effective research.
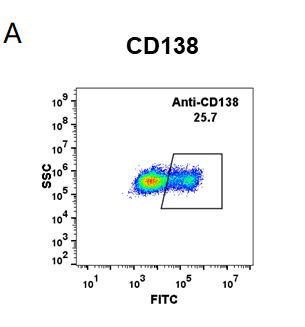
| Indatuximab (Anti-CD138) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | CD138 |
| Reactivity: | Human |
How Does the Indatuximab Biosimilar Compare?
Indatuximab biosimilar offers researchers a high-quality, research-use-only alternative to the original drug. It mirrors the structural and functional attributes of Indatuximab ravtansine, ensuring consistency and reliability in preclinical studies.
Benefits of the Indatuximab Biosimilar
- Research Applications: Enables in-depth exploration of CD138-related pathways and ADC mechanisms.
- Cost-Effective: Provides an accessible solution for academic and industrial researchers.
- High Quality: Manufactured to meet stringent standards for preclinical use.
Disclaimer:
The Indatuximab biosimilar is for research use only and is not intended for diagnostic or therapeutic purposes.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Marina Alberto, PhD
Marina Alberto, PhD, holds a robust academic background in Biotechnology, earning her Bachelor’s Degree and PhD in Science and Technology from Quilmes National University. Her research spans cancer immunotherapy, glycan profiling, and vaccine development, including innovative projects on pediatric leukemia diagnosis and cancer-associated carbohydrate-mimetic vaccines. She currently serves as a Technical Support and Sales Specialist at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025