Imalumab: Unveiling Its Mechanism, Clinical Potential, and Biosimilar Advancements
What You Need to Know About Imalumab
What is Imalumab?
Imalumab is a monoclonal antibody targeting macrophage migration inhibitory factor (MIF), a key regulator of inflammation and immune response in cancer and other diseases.
How Does Imalumab Work?
Imalumab binds to MIF, reducing pro-inflammatory signaling pathways that contribute to tumor progression and immune evasion.
What Are the Clinical Applications of Imalumab?
Emerging research suggests Imalumab's potential in oncology, particularly for colorectal and ovarian cancers, due to its role in modulating the tumor microenvironment.
1.) Understanding Imalumab
Imalumab represents an innovative approach in the realm of immunotherapy, specifically targeting macrophage migration inhibitory factor (MIF), a cytokine that plays a critical role in promoting inflammation and tumor progression. MIF is implicated in a variety of pathological processes, including cancer, where its overexpression has been linked to poor prognosis across numerous malignancies. In solid tumors, high MIF levels are associated with increased tumor cell survival, enhanced angiogenesis, and a suppression of immune responses, all of which facilitate tumor growth and metastasis.
By inhibiting MIF, Imalumab seeks to block these detrimental processes, providing a new strategy to fight cancer and chronic inflammatory diseases. The therapeutic potential of Imalumab is not limited to oncology alone. It holds promise in treating autoimmune and inflammatory conditions, such as rheumatoid arthritis, where MIF plays a pivotal role in sustaining the inflammatory response. Furthermore, its applications extend to fibrosis, another disease driven by dysregulated inflammation and tissue scarring. Research suggests that Imalumab may have a broad impact on these diseases, offering hope for improved outcomes through MIF-targeted therapies.
The significance of Imalumab is further underscored by its ability to modulate the immune system's response. By blocking MIF’s interaction with its receptors, such as CD74 and CXCR2, it has the potential to reshape the immune microenvironment, facilitating a stronger, more effective immune response against both cancerous and inflammatory cells. As clinical trials continue, the role of Imalumab in precision medicine—tailoring treatment to individual patients based on specific biomarkers—is expected to expand, potentially offering a more personalized and effective approach to treatment.
2.) Mechanism of Action of Imalumab
Imalumab’s mechanism of action revolves around the inhibition of macrophage migration inhibitory factor (MIF), a critical cytokine involved in a wide range of inflammatory and tumorigenic processes. MIF interacts with its receptors, CD74 and CXCR2, to activate a variety of downstream signaling pathways that promote tumor survival, immune suppression, and chronic inflammation. These interactions are particularly important in the context of cancer, where MIF facilitates immune evasion by tumor cells, enabling them to thrive in hostile environments.
By blocking the binding of MIF to its receptors, Imalumab disrupts these signaling pathways, leading to the downregulation of pro-survival mechanisms within cancer cells. This inhibition not only enhances the immune system’s ability to target and eliminate tumor cells but also helps reverse the immunosuppressive tumor microenvironment. One of the key effects of MIF inhibition is the modulation of macrophage polarization, shifting macrophages from a pro-tumorigenic, immune-suppressive phenotype to an anti-tumor phenotype. This change boosts the recruitment and activation of immune cells that can fight cancer, such as cytotoxic T cells and natural killer (NK) cells.
In addition to its anti-cancer effects, the disruption of MIF signaling can also reduce chronic inflammation, which is a hallmark of many autoimmune diseases and conditions like rheumatoid arthritis and fibrosis. Imalumab’s ability to target MIF, thereby altering immune cell function and inflammation pathways, positions it as a potential game-changer in both cancer and chronic inflammatory disease therapies. As research progresses, the full extent of its therapeutic benefits in these areas will continue to unfold.
3.) Clinical Applications of Imalumab
The clinical applications of Imalumab are currently being explored across a range of cancer types and inflammatory diseases, with promising preclinical and early-stage clinical data highlighting its potential. In oncology, Imalumab’s ability to inhibit macrophage migration inhibitory factor (MIF) holds great promise for improving treatment outcomes in cancers that are characterized by immune evasion and inflammation. One key area of interest is colorectal cancer, where MIF is known to contribute to chemoresistance. Studies suggest that by blocking MIF signaling, Imalumab could enhance the efficacy of chemotherapy and immunotherapy, potentially overcoming resistance mechanisms and improving response rates in this patient population.
Imalumab’s application extends beyond colorectal cancer, with preclinical models showing encouraging results in ovarian cancer. MIF’s role in suppressing immune cell infiltration into tumors is well-documented, and Imalumab’s potential to promote immune cell entry into tumor sites could significantly enhance immune responses against ovarian cancer. Additionally, ongoing research is investigating Imalumab’s effects on lung cancer, where tumor-associated inflammation often accelerates disease progression. By targeting MIF, Imalumab may help reduce this inflammation, thereby counteracting one of the key drivers of lung cancer's aggressive nature.
Despite some setbacks in clinical trials, interest in MIF-targeting therapies like Imalumab remains high, with researchers continuing to refine treatment regimens. Ongoing studies are focusing on optimizing combination therapies, pairing Imalumab with other immune checkpoint inhibitors or traditional cancer treatments, to improve patient outcomes. As more data emerges, Imalumab may become a cornerstone of treatment for cancers and inflammatory diseases, offering a novel way to harness the immune system for therapeutic benefit.
4.) Exploring Biosimilars for Imalumab
What is a Biosimilar?
Biosimilars are biologic products that are highly similar to an existing reference drug, with no clinically meaningful differences in safety, purity, or potency. They provide cost-effective alternatives for research and therapeutic applications.
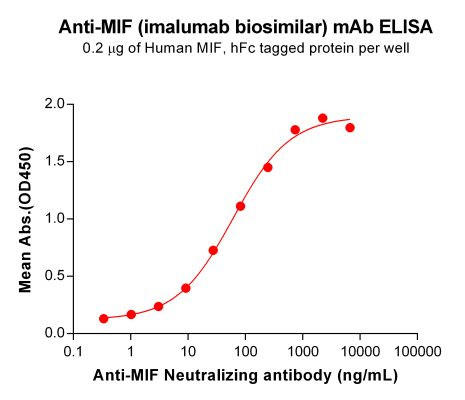
| Imalumab (Anti-MIF) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | MIF |
| Reactivity: | Human |
How Does the Imalumab Biosimilar Compare to Imalumab?
The Imalumab biosimilar retains the same mechanism of action, targeting MIF with high specificity. While not approved for clinical use, biosimilars serve as crucial tools in preclinical studies, enabling researchers to explore new therapeutic avenues.
Benefits of the Imalumab Biosimilar
- Research Accessibility: Enables broader study of MIF-targeting strategies.
- Cost-Effective Alternative: Reduces expenses associated with drug development.
- Reproducibility: Ensures consistent results in laboratory investigations.
Research Use Only Disclaimer:
The Imalumab biosimilar is intended solely for research applications and is not approved for clinical use.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Chris McNally, PhD
Chris McNally, PhD, has a strong foundation in Biomedical Science, completing a PhD scholarship in collaboration with Randox Laboratories and Ulster University. Chris has published extensively in prostate cancer research, focusing on biomarker discovery, cancer risk stratification, and molecular mechanisms such as hypoxia-induced regulation. He currently serves as a Business Development Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



