Icatolimab: Revolutionizing the Fight Against Cancer via CD47 Targeting
Quick Facts About Icatolimab
What is Icatolimab?
Icatolimab is a monoclonal antibody designed to target CD47, a protein that cancer cells use to evade immune detection.
How does Icatolimab work?
By blocking CD47, Icatolimab enables macrophages to recognize and eliminate cancer cells, enhancing the immune system's response.
What are the clinical applications of Icatolimab?
Icatolimab is primarily investigated for treating hematological malignancies and solid tumors, with promising results in emerging studies.
1.) Understanding Icatolimab
Icatolimab represents a significant advancement in immuno-oncology, focusing on the immune checkpoint CD47. CD47 functions as a "don’t eat me" signal, allowing cancer cells to evade immune system detection by binding to signal regulatory protein alpha (SIRPα) on macrophages. This inhibitory interaction prevents phagocytosis, allowing tumor cells to proliferate and spread unchecked. Icatolimab targets this pathway, blocking the CD47-SIRPα interaction, thereby removing the “don’t eat me” signal and enabling macrophages to recognize and engulf malignant cells, restoring immune surveillance.
The therapeutic potential of Icatolimab lies in its ability to bridge innate and adaptive immunity. While CD47 inhibition is not a novel concept in cancer immunotherapy, Icatolimab offers unique pharmacological properties that improve specificity and minimize toxicity compared to earlier candidates. Its innovative design reduces off-target effects, particularly the risk of widespread red blood cell phagocytosis, which has been a concern with first-generation CD47 inhibitors. This enhanced selectivity and reduced systemic toxicity enhance Icatolimab's safety profile, making it a more viable and sustainable treatment option for cancer patients.
Emerging studies highlight Icatolimab’s promising efficacy in a broad range of cancers, from hematologic malignancies such as lymphomas to solid tumors like colorectal, ovarian, and lung cancers. Preclinical and early clinical data suggest that Icatolimab could significantly improve tumor response rates, especially when used in combination with other immune checkpoint inhibitors, targeted therapies, or chemotherapy. As researchers continue to explore its potential, Icatolimab’s development could play a pivotal role in the growing field of immune-based cancer treatments. Its ability to harness both innate and adaptive immune responses positions it as a key player in the next generation of immuno-oncology therapies, offering the potential for durable anti-tumor responses and improved patient outcomes.
2.) Mechanism of Action of Icatolimab
Icatolimab works by blocking the interaction between CD47 on cancer cells and signal-regulatory protein alpha (SIRPα) on macrophages. CD47, known as a "don’t eat me" signal, helps tumor cells avoid immune system detection. By disrupting this interaction, Icatolimab allows macrophages to recognize and phagocytose cancer cells, thereby promoting their destruction. This direct effect on innate immunity is further complemented by Icatolimab’s ability to indirectly stimulate an adaptive immune response. As macrophages engulf cancer cells, they release tumor antigens, which are subsequently presented to T cells, triggering a broader immune response and creating a comprehensive anti-tumor effect.
Icatolimab's unique mechanism addresses two critical challenges in oncology: immune evasion by tumor cells and the immunosuppressive tumor microenvironment. Cancer cells often exploit the CD47-SIRPα pathway to evade immune surveillance, contributing to unchecked tumor growth. Icatolimab’s precise targeting of CD47 ensures that this evasion is interrupted without harming healthy cells, which reduces the risk of common side effects such as anemia, a problem often seen with CD47 blockade therapies.
Emerging clinical data suggest that Icatolimab may also synergize with other treatments, such as immune checkpoint inhibitors, to enhance its anti-cancer efficacy. By combining Icatolimab with existing therapies, researchers are exploring ways to overcome treatment resistance and relapse in cancers that are difficult to treat. This combination approach has the potential to redefine treatment protocols for a wide range of cancers, offering hope for patients with relapsed or resistant tumors.
3.) Clinical Applications of Icatolimab
Icatolimab is at the forefront of therapies aimed at combating hematological malignancies, such as non-Hodgkin’s lymphoma and multiple myeloma, as well as solid tumors like ovarian and colorectal cancers. This novel immunotherapy is gaining recognition for its ability to target the CD47-SIRPα immune evasion pathway, which is commonly exploited by cancer cells to avoid immune system detection. By blocking this interaction, Icatolimab enhances macrophage-mediated phagocytosis and facilitates the recognition and destruction of tumor cells, offering a promising approach for various cancer types.
Emerging clinical trials show promising results, with Icatolimab demonstrating efficacy in reducing tumor burden and prolonging survival rates in both hematological and solid tumors. What sets Icatolimab apart is its consistent performance across diverse cancer types, making it a versatile and adaptable tool in oncology. Its ability to generate durable anti-tumor responses across a broad range of malignancies offers new hope for patients with cancers that are difficult to treat with traditional therapies.
The drug’s safety profile has also been a focal point in its development. While traditional CD47-targeting therapies have been associated with a high incidence of cytopenia and other hematological side effects, Icatolimab’s refined design minimizes such risks, ensuring better patient tolerance. This reduction in side effects makes Icatolimab a more viable treatment option for long-term cancer care.
Furthermore, its potential in combination therapies has garnered significant attention. Preclinical and clinical studies suggest that Icatolimab, when used alongside PD-1/PD-L1 inhibitors, can produce synergistic effects, further enhancing its anti-cancer efficacy. This combination approach offers hope for patients with refractory cancers, potentially improving outcomes in difficult-to-treat malignancies.
4.) Advancing Research on Icatolimab with Biosimilars
What is a Biosimilar?
A biosimilar is a biologic product designed to be highly similar to an already approved reference product, with no clinically meaningful differences in safety, purity, or potency. Biosimilars are invaluable in research, enabling broader access to cutting-edge therapies.
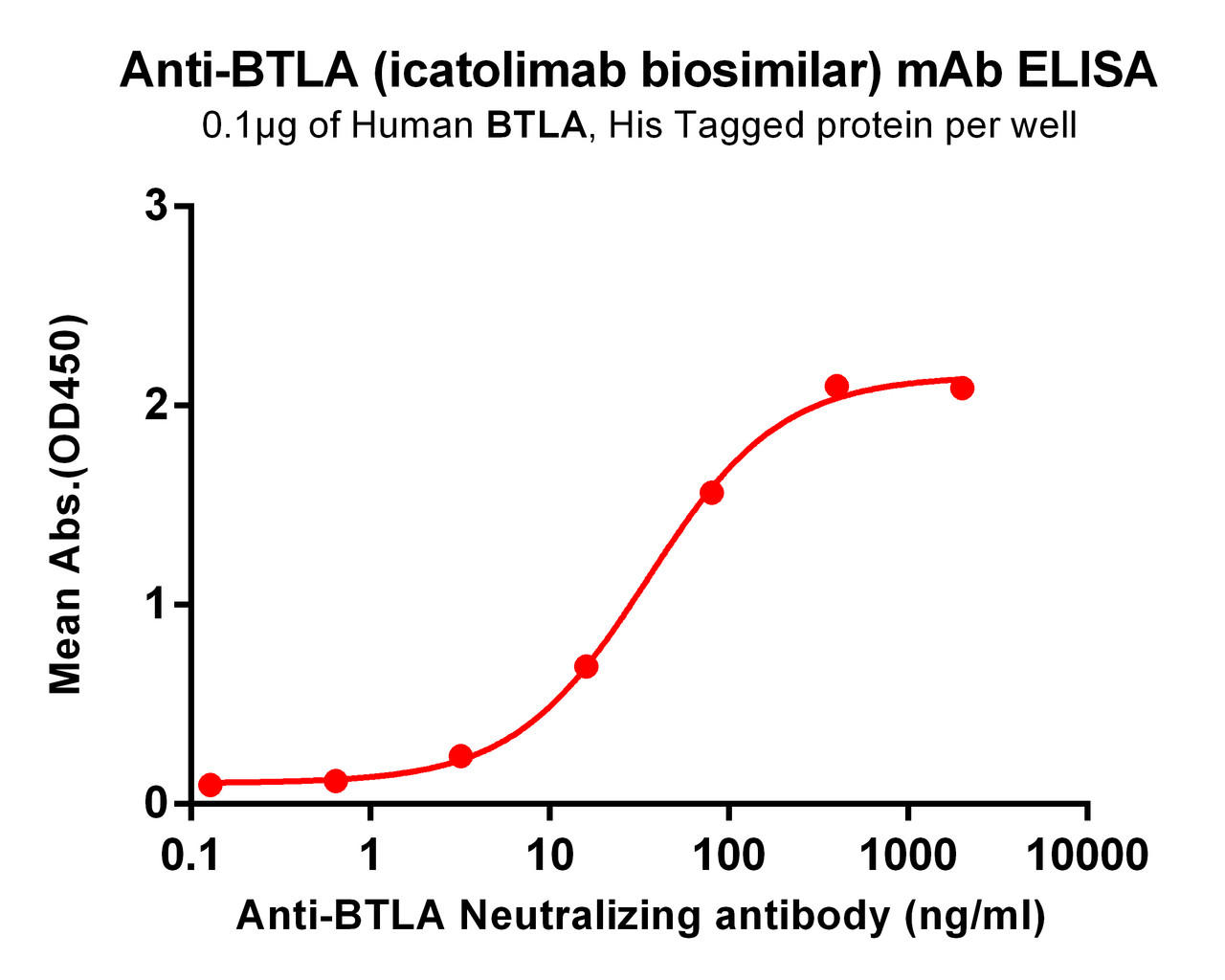
| Icatolimab (Anti-BTLA) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | BTLA |
| Reactivity: | Human |
How Do Icatolimab Biosimilars Compare?
Icatolimab biosimilars maintain the core functionalities of the original molecule, offering researchers a cost-effective option for preclinical and translational studies. These biosimilars provide an opportunity to explore novel applications and combinations without the high cost of proprietary drugs.
Benefits of Icatolimab Biosimilars
- Cost Efficiency: Biosimilars reduce the financial barriers to conducting extensive research.
- Expanded Access: They allow more labs to explore CD47-targeted therapies, accelerating discovery.
- Regulatory Pathways: As research tools, these biosimilars adhere to "research use only" guidelines, facilitating exploratory studies without the complexities of clinical-grade compliance.
Research Use Only Disclaimer:
Icatolimab biosimilars are for research use only and are not intended for clinical applications.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Miren Ruiz de Eguilaz, PhD
Miren Ruiz de Eguilaz, PhD, has an extensive academic background, earning a BSc in Biology from UPV/EHU, an MSc in Biotechnology from the University of Oviedo, and a PhD in Chemistry from Dublin City University (DCU). Miren’s expertise lies in biosensor technology and bacterial diagnostics. She currently serves as a Product Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Praluzatamab: Unveiling the Promise of CD47-Targeted Therapy in Cancer Research
Quick Facts About PraluzatamabWhat is Praluzatamab?Praluzatamab is an experimental mon …13th May 2025



