Ianalumab: Exploring Its Mechanism, Clinical Applications, and Research Potential
Quick Facts About Ianalumab
What is Ianalumab?
Ianalumab is a monoclonal antibody currently being investigated for its potential in treating autoimmune diseases, particularly lupus and immune thrombocytopenia (ITP). It is developed to target and inhibit B-cell activation, a critical mechanism in autoimmune responses.
What is the mechanism of action for Ianalumab?
Ianalumab works by targeting and inhibiting the B-lymphocyte stimulator (BLyS), which is essential for the survival and activation of B cells. By blocking BLyS, it reduces the number of autoreactive B cells responsible for autoimmune damage.
What are the clinical applications of Ianalumab?
Ianalumab has shown promise in treating autoimmune diseases such as lupus, lupus nephritis, and ITP. Ongoing research also explores its potential for other autoimmune disorders like Sjögren's syndrome.
1.) Understanding Ianalumab
Ianalumab is a monoclonal antibody being investigated for its potential to treat autoimmune diseases, such as lupus, immune thrombocytopenia (ITP), and Sjögren's syndrome. It specifically targets B lymphocyte stimulator (BLyS), a cytokine that is integral to the survival and activation of B cells. In autoimmune diseases, B cells become dysregulated and produce autoantibodies that attack the body’s tissues, causing inflammation and damage. By inhibiting BLyS, Ianalumab helps prevent the survival of these autoreactive B cells, effectively reducing the autoimmune response.
The role of Ianalumab extends beyond merely suppressing immune activity; it aims to regulate and normalize B cell function, which is crucial for the treatment of autoimmune conditions. This regulatory approach differs from traditional immune suppression therapies, which often dampen the entire immune system and may leave patients vulnerable to infections. Ianalumab’s targeted action offers a more precise method of managing autoimmune diseases, potentially providing long-term relief with fewer side effects.
Currently under clinical investigation, Ianalumab shows promise in treating diseases where B cell dysregulation plays a key role. Its potential benefits for patients with conditions like lupus nephritis and ITP make it a promising candidate for expanding therapeutic options in autoimmune disease management. Ongoing research will continue to define its precise role in clinical practice, with the goal of offering an effective, targeted treatment for patients who have not responded to conventional therapies.
2.) Mechanism of Action of Ianalumab
The primary action of Ianalumab involves its ability to block the B lymphocyte stimulator (BLyS) cytokine, which is essential for the survival, maturation, and activation of B cells. In healthy individuals, BLyS helps maintain the balance of immune function by supporting B cell activity when necessary. However, in autoimmune diseases, an overproduction of BLyS leads to the proliferation of autoreactive B cells, which produce antibodies that attack the body’s own tissues.
By binding to BLyS, Ianalumab prevents it from interacting with its receptors on B cells. This inhibition results in the reduced survival and activation of these B cells, which, in turn, decreases the production of harmful autoantibodies. As a result, Ianalumab helps restore immune system balance by regulating B cell activity and reducing the inflammatory responses that drive autoimmune diseases.
This targeted mechanism offers a distinct advantage over traditional immunosuppressive therapies, which can indiscriminately affect the entire immune system. Ianalumab’s selective inhibition of BLyS provides a more precise and controlled approach to managing autoimmune disorders. By focusing on the root cause of the problem - dysregulated B cell activity - this treatment can potentially provide relief from symptoms while minimizing the risk of broad immune suppression.
As research on Ianalumab continues, its mechanism of action promises to be a crucial component in the development of more effective treatments for autoimmune diseases, offering patients a safer and more targeted alternative.
3.) Clinical Applications of Ianalumab
Ianalumab holds significant promise in the treatment of several autoimmune diseases, especially those where B cell dysregulation plays a pivotal role. The drug has shown particular promise in systemic lupus erythematosus (SLE), a chronic autoimmune disorder characterized by widespread inflammation and tissue damage, particularly in organs like the kidneys. In lupus nephritis, a severe complication of lupus, Ianalumab’s ability to modulate B cell activity could lead to better disease control, reduce proteinuria (a key marker of kidney damage), and improve kidney function.
Beyond lupus, Ianalumab is being investigated for its potential in treating immune thrombocytopenia (ITP), a condition where the immune system mistakenly targets and destroys platelets. By reducing the number of autoreactive B cells, Ianalumab helps maintain platelet levels, which is crucial for preventing bleeding complications in ITP patients. The drug’s effect on B cells also opens up the possibility of treating other autoimmune diseases, such as Sjögren’s syndrome, where similar mechanisms of B cell dysfunction drive disease progression.
Ongoing clinical trials are exploring the full potential of Ianalumab, with emerging studies suggesting that it may offer a valuable treatment option for patients who have not responded to conventional therapies. The targeted nature of Ianalumab’s mechanism, focusing on B cell regulation rather than broad immune suppression, may provide a safer and more effective approach to managing these chronic and often debilitating autoimmune diseases. As clinical research progresses, Ianalumab’s role in the therapeutic landscape is expected to expand, offering hope for improved outcomes in patients with autoimmune conditions.
4.) Advancing Research on Ianalumab Biosimilars
What is a Biosimilar?
A biosimilar is a biologic product that is highly similar to an already approved reference biologic drug, with no clinically meaningful differences in terms of safety, purity, or potency. Biosimilars offer a cost-effective alternative and are particularly valuable in advancing medical research by providing access to drugs for clinical trials and exploratory studies without the high costs of the original biologics.
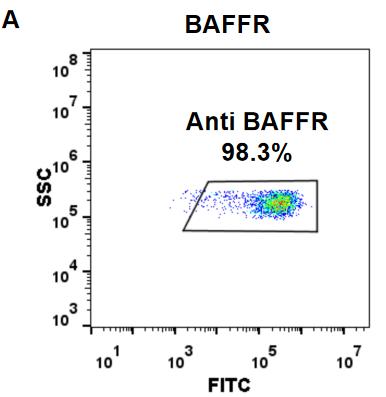
| Ianalumab (Anti-BAFFR) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | BAFF-R |
| Reactivity: | Human |
How Ianalumab Biosimilar Compares to Ianalumab
Ianalumab biosimilar shares the same mechanism of action as the original Ianalumab, targeting and inhibiting the BLyS cytokine to reduce abnormal B cell activity in autoimmune diseases. It is designed to be therapeutically equivalent, providing researchers with a more affordable option for studying the drug's effects across various disease models. While the biosimilar is structurally similar to the original, its production may involve slightly different processes, yet it has been shown to be equally effective in research settings.
Benefits of Ianalumab Biosimilar
Research Use Only Disclaimer:
Our biosimilar product,is available for research use only. It provides a valuable resource for scientific investigations, helping researchers expand the potential therapeutic applications of Ianalumab.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Chris McNally, PhD
Chris McNally, PhD, has a strong foundation in Biomedical Science, completing a PhD scholarship in collaboration with Randox Laboratories and Ulster University. Chris has published extensively in prostate cancer research, focusing on biomarker discovery, cancer risk stratification, and molecular mechanisms such as hypoxia-induced regulation. He currently serves as a Business Development Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



