Fezakinumab: Exploring Its Mechanism and Clinical Potential
Quick Facts About Fezakinumab
What is Fezakinumab?
Fezakinumab is a human IgG1-lambda monoclonal antibody that targets interleukin-22 (IL-22), a cytokine involved in inflammatory responses.
What is the mechanism of action for Fezakinumab?
By binding to IL-22, Fezakinumab inhibits its activity, thereby reducing inflammation associated with various diseases.
What are the clinical applications of Fezakinumab?
Fezakinumab has been investigated in clinical trials for conditions such as atopic dermatitis and rheumatoid arthritis.
1.) Understanding Fezakinumab
The development of Fezakinumab was initiated by Wyeth Pharmaceuticals and later continued by Pfizer following their acquisition of Wyeth. As a fully human monoclonal antibody targeting interleukin-22 (IL-22), Fezakinumab has been investigated for its potential to regulate immune responses and mitigate inflammation in various disease states. IL-22 plays a crucial role in immune defense and tissue homeostasis, particularly in epithelial barriers such as the skin, lungs, and gastrointestinal tract. However, excessive IL-22 signaling has been implicated in several inflammatory and autoimmune conditions, making it a promising therapeutic target.
One of the primary areas of interest for Fezakinumab has been its application in dermatological and autoimmune diseases. Clinical studies have evaluated its efficacy in atopic dermatitis, where IL-22 contributes to epidermal thickening and barrier dysfunction. By neutralizing IL-22, Fezakinumab aims to reduce skin inflammation and restore epidermal integrity. Similarly, researchers have explored its role in treating rheumatoid arthritis (RA) and inflammatory bowel disease (IBD), conditions where IL-22-driven inflammation exacerbates tissue damage.
Beyond autoimmune disorders, Fezakinumab has been investigated for potential use in chronic obstructive pulmonary disease (COPD) and psoriasis, where dysregulated IL-22 signaling contributes to persistent inflammation. While early clinical trials showed promise, further large-scale studies are needed to determine its long-term efficacy and safety. Ongoing research continues to explore Fezakinumab’s role in modulating IL-22-driven pathologies, highlighting its potential as a novel therapeutic approach for inflammatory diseases.
2.) Mechanism of Action of Fezakinumab
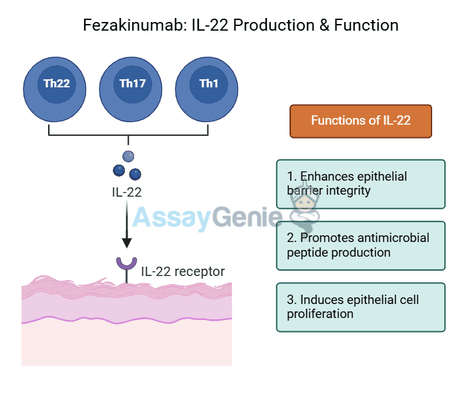
Fezakinumab functions as a fully human monoclonal antibody that specifically binds to interleukin-22 (IL-22), thereby preventing it from interacting with its receptor (IL-22R1/IL-10R2 heterodimer) on target cells. IL-22 is a key cytokine involved in tissue homeostasis and immune regulation, primarily influencing epithelial cells in barrier tissues such as the skin, lungs, and gastrointestinal tract. It plays a dual role: while it supports epithelial integrity, wound healing, and antimicrobial defense, excessive IL-22 signaling has been implicated in chronic inflammatory and autoimmune diseases.
Under normal physiological conditions, IL-22 is produced by T-helper 22 (Th22) cells, Th17 cells, natural killer (NK) cells, and innate lymphoid cells (ILCs). It promotes epithelial cell proliferation, survival, and secretion of antimicrobial peptides that enhance mucosal immunity. However, in dysregulated states, such as atopic dermatitis, rheumatoid arthritis (RA), psoriasis, and inflammatory bowel disease (IBD), elevated IL-22 levels drive excessive tissue remodeling, hyperplasia, and inflammation, contributing to disease pathology.
Fezakinumab exerts its therapeutic effect by selectively inhibiting IL-22, thus downregulating pro-inflammatory signaling without broadly suppressing the entire immune system. This targeted approach helps mitigate chronic inflammation while preserving key immune functions necessary for pathogen defense. Unlike general immunosuppressants that increase the risk of opportunistic infections, Fezakinumab fine-tunes immune activity, reducing disease symptoms while minimizing systemic side effects.
This mechanism makes Fezakinumab a promising therapeutic candidate for autoimmune and inflammatory conditions, where selective cytokine inhibition could offer greater safety and efficacy compared to traditional immunosuppressive treatments.

3.) Clinical Applications of Fezakinumab
Fezakinumab has undergone clinical evaluation for multiple inflammatory and autoimmune diseases, with a particular focus on conditions where interleukin-22 (IL-22) plays a key role in disease pathogenesis. Clinical trials have demonstrated promising results, particularly in atopic dermatitis and rheumatoid arthritis, although further studies are needed to establish its full therapeutic potential.
Atopic Dermatitis (AD)
A Phase 2a randomized, double-blind, placebo-controlled trial investigated the efficacy and safety of Fezakinumab in adults with moderate-to-severe atopic dermatitis who had an inadequate response to conventional therapies. The study found that Fezakinumab was well-tolerated and provided sustained clinical improvement even after the last dose was administered. These findings suggest that IL-22 blockade may play a significant role in reducing skin inflammation and restoring epithelial barrier function, which are key challenges in AD management. Given these positive results, Fezakinumab has been considered a potential targeted therapy for patients who do not respond well to existing biologics or topical treatments.
Rheumatoid Arthritis (RA)
Fezakinumab also progressed to Phase 2 clinical trials for rheumatoid arthritis (RA), an autoimmune disorder characterized by chronic joint inflammation and damage. While detailed trial outcomes remain limited, the interest in IL-22 as a therapeutic target underscores the potential role of Fezakinumab in modulating autoimmune inflammation beyond skin-related diseases.
Despite these encouraging findings, further research is necessary to fully assess Fezakinumab’s long-term safety, efficacy, and optimal dosing across different patient populations and disease settings. Large-scale Phase 3 trials and real-world studies will be essential to determine its clinical viability and regulatory approval for widespread therapeutic use.
4.) Exploring Biosimilars for Fezakinumab
As the development of biologic therapies like Fezakinumab progresses, the emergence of biosimilars offers new avenues for research and therapeutic applications.
What is a Biosimilar?
A biosimilar is a biologic medical product highly similar to an already approved reference product, with no clinically meaningful differences in terms of safety, purity, and potency. Biosimilars are developed to provide more accessible treatment options and to support ongoing research by offering comparable alternatives to existing biologics.
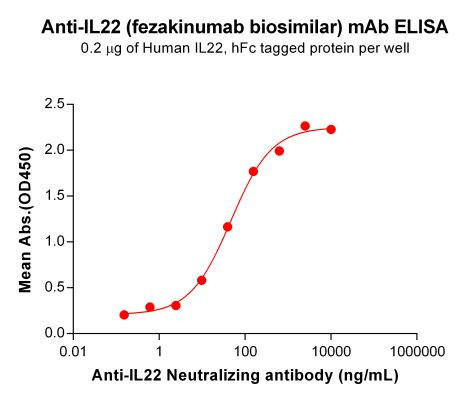
| Fezakinumab (Anti-IL22) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | IL-22 |
| Reactivity: | Human |
Comparison:
Biosimilars to Fezakinumab are designed to closely replicate the original antibody's structure and function, targeting IL-22 in the same manner. While minor differences in manufacturing processes may exist, these do not affect the clinical performance of the biosimilar.
Benefits:
In research settings, Fezakinumab biosimilars serve as valuable tools for studying IL-22-related pathways and diseases. They provide a cost-effective alternative for experimental purposes, facilitating broader investigation into inflammatory mechanisms and potential therapeutic interventions.
Research Use Only Disclaimer:
It's important to note that Fezakinumab biosimilars intended for research are designated for investigational use only. They are not approved for clinical application in humans and should be utilized solely within the scope of scientific research.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Marina Alberto, PhD
Marina Alberto, PhD, holds a robust academic background in Biotechnology, earning her Bachelor’s Degree and PhD in Science and Technology from Quilmes National University. Her research spans cancer immunotherapy, glycan profiling, and vaccine development, including innovative projects on pediatric leukemia diagnosis and cancer-associated carbohydrate-mimetic vaccines. She currently serves as a Technical Support and Sales Specialist at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025