Erenumab: Transforming Migraine Prevention Through CGRP Receptor Inhibition
Quick Facts About Erenumab
What is Erenumab?
Erenumab is a fully human monoclonal antibody designed to prevent migraines by targeting the calcitonin gene-related peptide (CGRP) receptor.
How does Erenumab work?
It binds selectively to the CGRP receptor, inhibiting its function and thereby reducing migraine frequency.
What are the clinical applications of Erenumab?
Erenumab is primarily used for the preventive treatment of migraines in adults.
1.) Understanding Erenumab
Erenumab represents a significant advancement in migraine therapy as the first FDA-approved treatment specifically designed to prevent migraines by inhibiting the calcitonin gene-related peptide (CGRP) receptor. Developed collaboratively by Amgen and Novartis, it is marketed under the brand name Aimovig. Unlike traditional migraine treatments that primarily focus on symptom relief, Erenumab addresses the underlying pathophysiology by targeting the CGRP pathway, which plays a crucial role in migraine attacks, including the dilation of blood vessels and increased pain signaling.
Administered via monthly subcutaneous injections, Erenumab offers a convenient and effective option for patients seeking to reduce the frequency, duration, and severity of migraine episodes. Its development underscores a shift towards mechanism-based therapies in neurology, providing a tailored approach that enhances efficacy while minimizing adverse effects commonly associated with broader-acting medications. Unlike beta-blockers, anticonvulsants, or tricyclic antidepressants—often prescribed for migraine prevention—Erenumab specifically blocks CGRP receptors without significant systemic side effects like dizziness, drowsiness, or weight gain.
Clinical trials have demonstrated its effectiveness, with many patients experiencing a substantial reduction in monthly migraine days. For individuals who have not responded well to conventional treatments, Erenumab provides an alternative with a well-tolerated safety profile. Its targeted mechanism and ease of use make it a groundbreaking option in migraine management, improving the quality of life for many sufferers.
2.) Mechanism of Action of Erenumab
Erenumab functions by selectively binding to the calcitonin gene-related peptide (CGRP) receptor, a crucial component in the transmission of migraine-related pain signals. CGRP is a neuropeptide that plays a central role in migraine pathophysiology, contributing to neurogenic inflammation, vasodilation, and pain sensitization. During a migraine attack, CGRP levels rise significantly, leading to the dilation of blood vessels and the activation of trigeminal nociceptive pathways, which results in headache pain.
By inhibiting the CGRP receptor, Erenumab prevents CGRP from exerting its vasodilatory effects and stops the cascade of events that lead to migraine onset. This targeted mechanism of action directly addresses a key underlying factor in migraine development rather than merely alleviating symptoms. Unlike conventional migraine treatments such as beta-blockers, antidepressants, or anticonvulsants, which have broader effects on the nervous system and can cause side effects like drowsiness, dizziness, and fatigue, Erenumab acts specifically on the CGRP receptor without interfering with other physiological processes.
Administered as a once-monthly subcutaneous injection, Erenumab provides long-term receptor blockade, allowing patients to experience sustained migraine prevention. Clinical trials have demonstrated its efficacy in reducing migraine frequency, duration, and severity, making it a valuable treatment option, particularly for those who have not responded well to traditional therapies. By offering a precise, mechanism-based approach, Erenumab represents a significant advancement in migraine management, improving patient outcomes while minimizing systemic side effects.
3.) Clinical Applications of Erenumab
Erenumab is specifically indicated for the preventive treatment of migraines in adults and has demonstrated significant efficacy across diverse patient populations. Clinical trials have shown that monthly subcutaneous doses of 70 mg or 140 mg can effectively reduce the frequency, severity, and duration of migraine attacks. Studies indicate that patients receiving Erenumab experience a significant reduction in monthly migraine days compared to placebo, with some achieving a 50% or greater decrease in migraine frequency.
One of the most notable advantages of Erenumab is its ability to improve patients’ overall quality of life. By preventing migraine episodes, it helps reduce the burden of disability associated with migraines, allowing patients to engage more fully in daily activities, work, and social interactions. Additionally, Erenumab has been linked to a decreased reliance on acute migraine medications, including triptans and analgesics, which are often associated with medication overuse headaches when taken frequently.
Erenumab has demonstrated efficacy in both episodic and chronic migraine sufferers. Episodic migraine patients (those experiencing fewer than 15 headache days per month) have reported a substantial decline in migraine frequency, while those with chronic migraines (15 or more headache days per month) have also benefited from a meaningful reduction in headache days. Its favorable safety profile, characterized by minimal systemic side effects, makes it a well-tolerated and reliable long-term option for migraine prophylaxis.
Overall, Erenumab represents a significant advancement in migraine prevention, offering sustained efficacy and improving the daily lives of individuals affected by this debilitating neurological disorder.
4.) Exploring Biosimilars for Erenumab
As the patent lifecycle of Erenumab progresses, the development of biosimilars presents an opportunity to enhance research and accessibility in migraine treatment.
What is a Biosimilar?
A biosimilar is a biologic medical product highly similar to an already approved reference product, with no clinically meaningful differences in terms of safety, purity, and potency. Biosimilars undergo rigorous evaluation to ensure they match the reference product's quality and efficacy.
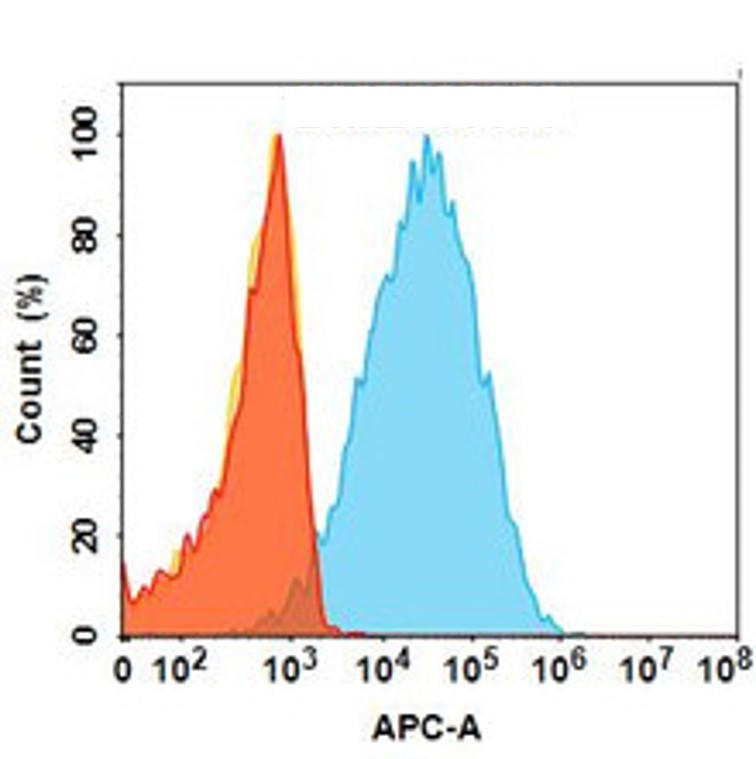
| Erenumab (Anti-CGRPR) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | CGRPR |
| Reactivity: | Human |
Comparison
Biosimilars of Erenumab are designed to replicate the therapeutic effects of the original monoclonal antibody, targeting the CGRP receptor to prevent migraines. While the active mechanisms remain consistent, biosimilars may offer variations in formulation or delivery methods.
Benefits
In research settings, biosimilars serve as valuable tools for studying migraine pathophysiology and developing new therapeutic strategies. Their availability can facilitate broader research initiatives and potentially lead to more cost-effective treatment options.
Research Use Only Disclaimer:
It's important to note that biosimilar products are intended for research purposes and may not be approved for clinical use. Researchers should ensure compliance with regulatory standards when utilizing these products in studies.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By David Lee, PhD
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Erenumab: Transforming Migraine Prevention Through CGRP Receptor Inhibition
Quick Facts About ErenumabWhat is Erenumab?Erenumab is a fully human monoclonal antibo …1st Apr 2025


