Enoblituzumab: Unveiling the Role of Anti-B7-H3 in Cancer Research
Key Facts About Enoblituzumab
What is Enoblituzumab?
Enoblituzumab is a monoclonal antibody targeting the B7-H3 protein, which is expressed on the surface of various cancer cells, including those in prostate cancer, sarcoma, and ovarian cancer.
What is the mechanism of action for Enoblituzumab?
Enoblituzumab works by binding to the B7-H3 protein on tumor cells, blocking its immune evasion mechanisms and enhancing immune system recognition and destruction of the cancer cells.
What are the clinical applications of Enoblituzumab?
Enoblituzumab is primarily being studied in clinical trials for its potential to treat cancers such as prostate cancer, sarcoma, and ovarian cancer. Emerging studies focus on its role in combination therapies.
1.) Understanding Enoblituzumab
Enoblituzumab is an innovative immunotherapeutic agent designed to target B7-H3, a protein that is often overexpressed on the surface of various tumor cells. B7-H3 belongs to the B7 family of immune checkpoint molecules, which are involved in regulating immune responses. However, in the context of cancer, B7-H3 plays a critical role in immune evasion by suppressing the activation of immune cells, particularly T-cells, thereby allowing tumors to escape immune surveillance and continue to proliferate.
The overexpression of B7-H3 in several malignancies, including prostate, melanoma, and lung cancer, makes it an attractive target for therapeutic intervention. By binding specifically to B7-H3, Enoblituzumab interferes with this immune suppressive pathway, reactivating the immune system and enhancing the ability of T-cells and other immune effector cells to recognize and attack tumor cells. This mechanism is crucial for overcoming the limitations of conventional therapies, particularly in cancers that are resistant to traditional treatments like chemotherapy and radiation.
What makes Enoblituzumab particularly promising is its ability to selectively target tumors that overexpress B7-H3 while sparing normal cells, which typically do not express this protein at high levels. This specificity could lead to more effective and less toxic treatment options for patients with advanced or resistant cancers. Additionally, ongoing research into combination therapies, where Enoblituzumab is paired with other immune checkpoint inhibitors or cancer treatments, shows potential to further enhance its clinical efficacy and broaden its applications in cancer immunotherapy.
Prefer to Listen? Check Out the Enoblituzumab Podcast Episode
2.) Mechanism of Action of Enoblituzumab
Enoblituzumab is an immunotherapeutic monoclonal antibody designed to target B7-H3, a protein overexpressed on various tumor cells, including those in prostate cancer and sarcoma. B7-H3 is part of the B7 family of immune checkpoint molecules and plays a crucial role in immune evasion by suppressing T-cell activity. This immune suppression allows tumors to evade detection and destruction by the immune system, facilitating tumor growth.
Enoblituzumab works by binding specifically to B7-H3 on tumor cells, inhibiting its ability to suppress immune responses. This blockage reactivates T-cells and other immune effector cells, enhancing the body's immune response against cancer cells. By disrupting the immune evasion mechanism, Enoblituzumab enables more effective targeting and elimination of tumor cells.
Additionally, Enoblituzumab shows promise in combination with other immune checkpoint inhibitors, such as pembrolizumab, which targets the PD-1 receptor on T-cells. Combining these therapies could provide a more comprehensive immune response, as Enoblituzumab addresses B7-H3-mediated immune suppression while pembrolizumab enhances T-cell activation. This dual targeting approach is a focus of ongoing research, with the goal of improving treatment outcomes for patients with cancers that are resistant to conventional therapies.

3.) Clinical Applications of Enoblituzumab
Clinical investigations into Enoblituzumab are exploring its application across various cancer types. Its promising results in prostate cancer, sarcoma, and ovarian cancer, along with its potential use in combination with other immunotherapies, make it a key subject of ongoing research. While FDA approval is still pending, its early-stage clinical trials have shown encouraging results.
Recent studies have shown that Enoblituzumab is safe and demonstrates promise in treating high-risk prostate cancers. Moreover, research is expanding into its use in other cancers, including Ewing sarcoma and ovarian cancer, where B7-H3 expression is significant.
Research is also expanding into other cancers, including Ewing sarcoma and ovarian cancer, where B7-H3 expression plays a significant role in tumor growth and immune evasion. Enoblituzumab's potential to be used in combination with other immunotherapies, such as immune checkpoint inhibitors, is being actively investigated. This combinatory approach could provide enhanced anti-tumor responses and broaden the drug’s clinical applications. While FDA approval is still pending, ongoing trials are positioning Enoblituzumab as a promising candidate in cancer immunotherapy.
4.) How Enoblituzumab Biosimilar Compares to Enoblituzumab
Our biosimilar product, Enoblituzumab Biosimilar, offers a valuable research tool in the study of anti-B7-H3 therapies. By offering a cost-effective and reliable option, this biosimilar enhances ongoing cancer research efforts without compromising the integrity of scientific findings.
What is a Biosimilar?
A biosimilar is a biologic medical product that is almost identical to an existing FDA-approved reference biologic, but it is developed and marketed once the original product's patent expires. Biosimilars undergo rigorous testing to ensure they match the reference in terms of quality, safety, and efficacy.
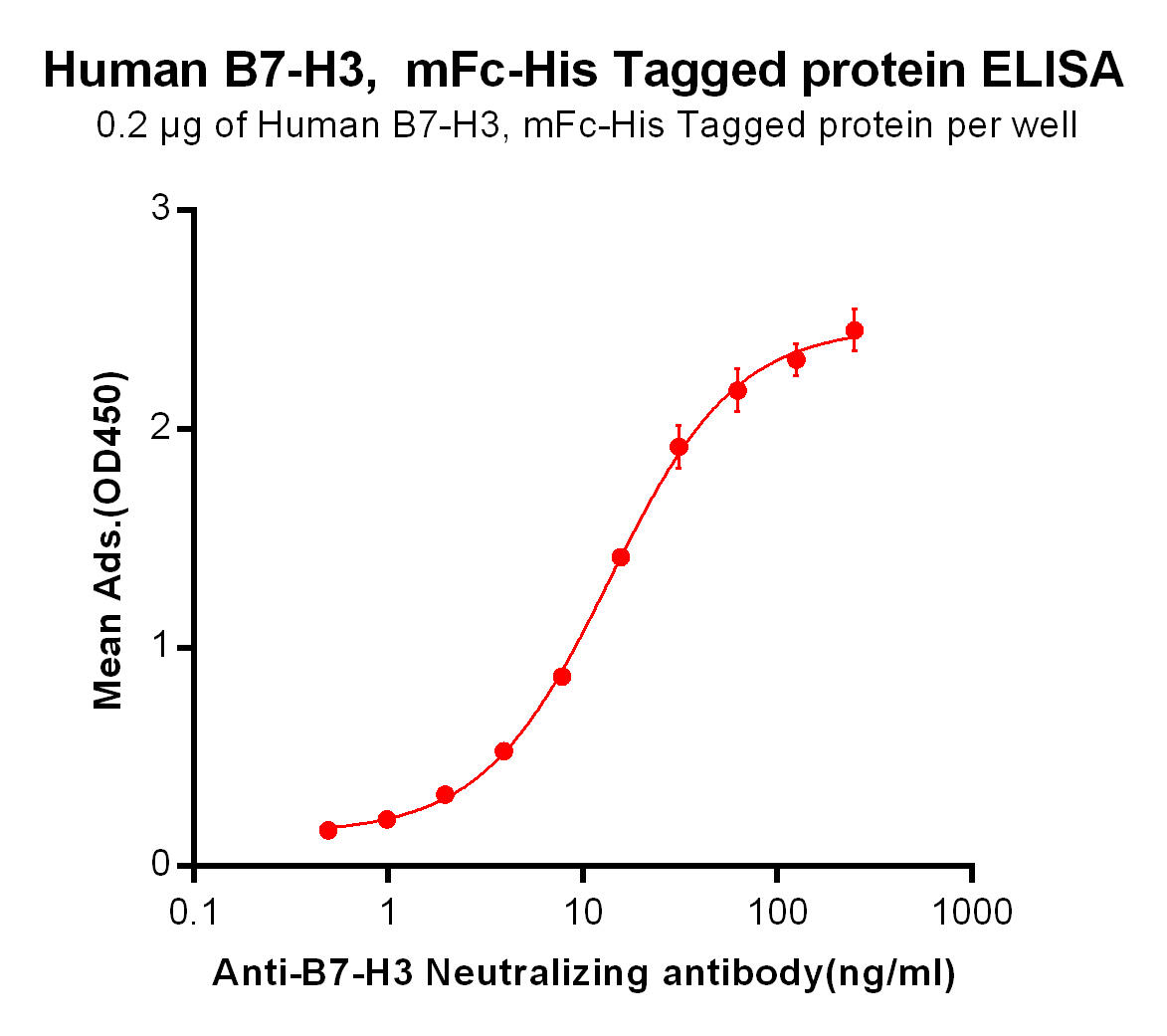
| Enoblituzumab (Anti-B7-H3) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | B7-H3 |
| Reactivity: | Human |
Comparison
The Enoblituzumab Biosimilar shares the same mechanism of action as the reference product, targeting the B7-H3 protein to enhance immune responses against cancer. It is designed to serve as a more accessible and cost-effective alternative for researchers studying the B7-H3 pathway in cancer treatment.
Benefits
The biosimilar provides significant advantages in research settings by allowing for large-scale testing without the prohibitive costs associated with the reference product. It supports a variety of research applications, particularly in the exploration of cancer immunotherapies.
Research Use Only Disclaimer
The Enoblituzumab Biosimilar is intended for research use only and is not approved for clinical or patient use.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Miren Ruiz de Eguilaz, PhD
Miren Ruiz de Eguilaz, PhD, has an extensive academic background, earning a BSc in Biology from UPV/EHU, an MSc in Biotechnology from the University of Oviedo, and a PhD in Chemistry from Dublin City University (DCU). Miren’s expertise lies in biosensor technology and bacterial diagnostics. She currently serves as a Product Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Erenumab: Transforming Migraine Prevention Through CGRP Receptor Inhibition
Quick Facts About ErenumabWhat is Erenumab?Erenumab is a fully human monoclonal antibo …1st Apr 2025



