Enfortumab Vedotin: Unveiling Its Role in Cancer Treatment and the Rise of Biosimilars
Quick Facts About Enfortumab
What is Enfortumab Vedotin?
Enfortumab vedotin is an innovative cancer therapy, primarily used in treating urothelial carcinoma (bladder cancer) that has not responded to prior chemotherapy.
What is the mechanism of action for Enfortumab?
Enfortumab vedotin targets Nectin-4, a protein found on cancer cells. It delivers a cytotoxic agent directly to these cells, resulting in cell death.
What are the clinical applications of Enfortumab?
Enfortumab vedotin is used for advanced or metastatic urothelial carcinoma, showing promise in combination with other therapies like pembrolizumab.
1.) Understanding Enfortumab Vedotin
Enfortumab vedotin is a breakthrough antibody-drug conjugate (ADC) that has gained recognition for its promising role in treating advanced urothelial carcinoma, particularly in patients who have experienced limited success with traditional chemotherapy regimens. This novel therapeutic approach combines the precision of monoclonal antibody targeting with the potent cytotoxic effects of chemotherapy agents. The drug works by targeting Nectin-4, a cell adhesion molecule that is highly expressed on the surface of urothelial carcinoma cells, especially those found in bladder cancer. By binding to Nectin-4, Enfortumab vedotin delivers a chemotherapy agent directly to the tumor, sparing surrounding healthy tissues and reducing the side effects commonly associated with conventional chemotherapy treatments.
The targeted delivery mechanism is one of the drug's most notable advantages, as it allows for the highly selective destruction of cancer cells. This specificity is particularly important in advanced cancers, where the ability to minimize damage to normal cells can significantly improve the patient's quality of life. Enfortumab vedotin has also shown promising results in combination with other immunotherapies, such as pembrolizumab, in ongoing clinical trials, making it an even more powerful weapon in treating metastatic urothelial carcinoma. These combination approaches aim to boost the immune system’s ability to fight cancer, which could result in enhanced efficacy and improved patient survival rates.
As a result of its mechanism and efficacy, Enfortumab vedotin has been approved by the FDA for use in patients who have previously received chemotherapy, offering a new avenue of treatment for those with advanced-stage bladder cancer. Ongoing research continues to explore its broader potential across other cancers and its use in combination therapies to increase its therapeutic benefits.
2.) Mechanism of Action of Enfortumab Vedotin
Enfortumab vedotin operates as an antibody-drug conjugate (ADC), a complex molecular therapy that combines three key components to target and treat cancer cells effectively. The first component is a monoclonal antibody that specifically binds to Nectin-4, a protein that is overexpressed on the surface of urothelial carcinoma cells. The presence of Nectin-4 in cancer cells, particularly in bladder cancer, makes it an ideal target for this therapeutic strategy. Once the monoclonal antibody binds to the Nectin-4 receptor, the entire ADC is internalized by the cancer cell, initiating a chain reaction that leads to tumor cell death.
The second component of Enfortumab vedotin is a linker that connects the monoclonal antibody to the cytotoxic agent. The linker is designed to remain stable in circulation, ensuring that the chemotherapy agent is only released once it has been delivered directly to the cancer cell. Inside the cell, the linker is cleaved, releasing the potent chemotherapy agent, which disrupts the microtubules within the cancer cell. This disruption halts the normal cell cycle, resulting in cell cycle arrest and ultimately triggering apoptosis, or programmed cell death.
By targeting cancer cells directly, Enfortumab vedotin minimizes the collateral damage to healthy cells that is often seen with traditional chemotherapy treatments. This highly selective mechanism of action allows for more effective tumor targeting with fewer side effects, making it an appealing alternative for patients with advanced urothelial carcinoma who have not responded to other therapies. Furthermore, research is investigating Enfortumab vedotin's potential synergy with other treatments, like checkpoint inhibitors, which could enhance its ability to stimulate an immune response against the tumor, potentially improving overall patient outcomes.
3.) Clinical Applications of Enfortumab Vedotin
Enfortumab vedotin has shown significant promise in the clinical setting, particularly for patients with advanced urothelial carcinoma who have not responded to traditional chemotherapy. Its most notable clinical application has been in patients with metastatic or locally advanced bladder cancer, where it has demonstrated robust efficacy in both single-agent use and in combination with other immunotherapies. One key clinical trial, the EV-301 trial, revealed that Enfortumab vedotin achieved a response rate of up to 40% in patients with chemotherapy-resistant bladder cancer, which was a remarkable improvement compared to traditional treatment options. As a result, Enfortumab vedotin has been approved by the U.S. FDA for use in patients who have previously undergone chemotherapy, offering these patients a new treatment option with proven efficacy.
In addition to its success in bladder cancer, Enfortumab vedotin is also being explored in combination with other therapies, such as the immune checkpoint inhibitor pembrolizumab. This combination therapy aims to enhance the immune system’s ability to recognize and destroy cancer cells, potentially improving survival rates and reducing tumor progression in patients with advanced or metastatic urothelial carcinoma. Clinical trials are ongoing to explore the full potential of this combination, and early results have been promising.
Beyond urothelial carcinoma, researchers are also investigating Enfortumab vedotin's potential in other cancers, including breast cancer, due to its mechanism of targeting specific tumor markers. The versatility of Enfortumab vedotin, combined with its efficacy in clinical trials, positions it as an important tool in the fight against cancer, with ongoing research potentially expanding its applications to a broader range of malignancies. As these studies progress, Enfortumab vedotin may continue to revolutionize cancer treatment by providing more effective and less toxic options for patients.
4.) Advancing Research on Enfortumab with Biosimilars
What is a Biosimilar?
A biosimilar is a biologic medical product highly similar to an existing FDA-approved reference product. While biosimilars are not identical, they are shown to have no clinically meaningful differences in terms of safety, purity, and potency. For researchers, biosimilars provide a cost-effective alternative for studying biologic therapies and can help expand the availability of treatments in clinical trials.
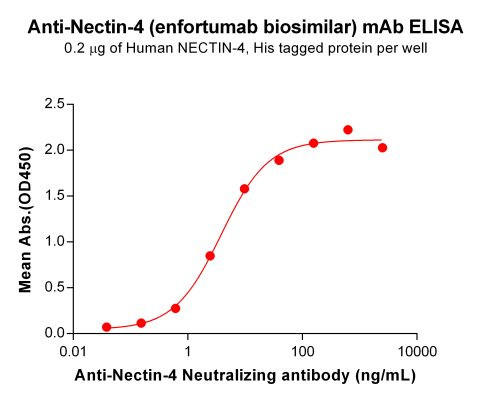
| Enfortumab (Anti-Nectin-4) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | NECTIN4 |
| Reactivity: | Human |
Comparison: Enfortumab Biosimilar vs. Enfortumab Vedotin
Enfortumab biosimilars are designed to mirror the action and therapeutic benefits of Enfortumab vedotin. Research use only biosimilars enable studies without the high costs of the reference product. While they offer similar efficacy, biosimilars may vary slightly in their formulation or manufacturing process, though such variations do not impact their effectiveness in preclinical or clinical research settings.
Benefits for Research
Research Use Only Disclaimer:
It is important to note that Enfortumab biosimilars are intended strictly for research purposes and not for direct patient administration.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Miren Ruiz de Eguilaz, PhD
Miren Ruiz de Eguilaz, PhD, has an extensive academic background, earning a BSc in Biology from UPV/EHU, an MSc in Biotechnology from the University of Oviedo, and a PhD in Chemistry from Dublin City University (DCU). Miren’s expertise lies in biosensor technology and bacterial diagnostics. She currently serves as a Product Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Erenumab: Transforming Migraine Prevention Through CGRP Receptor Inhibition
Quick Facts About ErenumabWhat is Erenumab?Erenumab is a fully human monoclonal antibo …1st Apr 2025


