Enapotamab: Advancing Cancer Research with Innovative Therapeutics
Quick Facts About Enapotamab
What is Enapotamab?
Enapotamab is an experimental antibody-drug conjugate (ADC) developed to target tumor cells selectively, offering new hope in cancer treatment.
How does Enapotamab work?
Enapotamab combines a monoclonal antibody with a cytotoxic agent to selectively bind to tumor-associated antigens, delivering a potent anti-cancer payload directly to cancer cells.
What are the clinical applications of Enapotamab?
This ADC has shown potential in treating solid tumors such as non-small cell lung cancer (NSCLC), melanoma, and head-and-neck cancers.
1.) Understanding Enapotamab
Enapotamab vedotin represents a groundbreaking advance in oncology therapeutics as an antibody-drug conjugate (ADC). ADCs are a sophisticated class of cancer therapies that integrate the specificity of monoclonal antibodies with the potent cytotoxic effects of chemotherapeutic agents. This dual-action mechanism allows ADCs like Enapotamab to precisely target tumor-associated antigens (TAAs), sparing healthy tissues and reducing systemic toxicity—a major limitation of traditional chemotherapy.
Developed by Genmab, Enapotamab was designed to address the challenges posed by aggressive and treatment-resistant cancers. By targeting TAAs such as AXL—a receptor tyrosine kinase overexpressed in several malignancies—Enapotamab specifically binds to cancer cells, inhibiting tumor progression while delivering its cytotoxic payload directly to malignant tissues. AXL is implicated in key oncogenic processes, including tumor cell proliferation, metastasis, immune evasion, and resistance to standard therapies, making it an ideal target for Enapotamab’s action.
Enapotamab’s mechanism of action underscores the principles of precision medicine, focusing on tailoring treatments to the molecular and genetic profiles of individual tumors. The conjugation of a potent cytotoxic agent to its monoclonal antibody ensures that cancer cells are selectively eradicated while minimizing harm to normal tissues. This targeted approach not only enhances the therapeutic efficacy of the treatment but also significantly improves the quality of life for patients by reducing the burden of treatment-related side effects.
Although clinical trials faced challenges, Enapotamab’s innovative design continues to inspire the development of next-generation ADCs. These advancements hold promise for expanding the therapeutic landscape in oncology and offering renewed hope for patients with resistant or aggressive cancers.

2.) Mechanism of Action of Enapotamab
The mechanism of action of Enapotamab is a sophisticated and highly targeted approach to cancer therapy, combining the precision of monoclonal antibody targeting with the potency of a cytotoxic payload. As an antibody-drug conjugate (ADC), Enapotamab is designed to selectively deliver its anti-cancer agent directly to tumor cells, thereby maximizing therapeutic efficacy while minimizing systemic toxicity.
The core of Enapotamab’s action involves two key components: a monoclonal antibody and a cytotoxic payload. The monoclonal antibody component is engineered to bind specifically to AXL, a receptor tyrosine kinase that is frequently overexpressed on the surface of various cancers, including non-small cell lung cancer (NSCLC), melanoma, and others. AXL plays a critical role in several tumorigenic processes, such as tumor growth, metastasis, immune evasion, and resistance to conventional therapies, making it an ideal target for Enapotamab’s action.
Upon administration, Enapotamab binds to the AXL receptors on the surface of tumor cells. This receptor-mediated binding triggers internalization of the ADC, where it is transported into the cell. Once inside, the cytotoxic payload is released. This payload, a potent anti-cancer agent, interferes with essential cellular processes such as DNA replication and cell division, leading to the activation of apoptotic pathways. As a result, the cancer cell undergoes programmed cell death (apoptosis), preventing further tumor growth.
What sets Enapotamab apart from traditional chemotherapies is its precision-targeted mechanism. By focusing on AXL-expressing cancer cells, it spares healthy, non-cancerous cells from the toxic effects of the cytotoxic agent. This selective targeting greatly reduces the risk of off-target side effects, which are common with conventional chemotherapy, thereby improving the patient’s quality of life and enhancing the therapeutic outcomes for aggressive cancers, particularly those with limited treatment options.
3.) Clinical Applications of Enapotamab
Enapotamab’s clinical applications are centered on its ability to target solid tumors, particularly those resistant to conventional therapies. As an antibody-drug conjugate (ADC), it combines the specificity of monoclonal antibodies with the potency of cytotoxic agents, making it effective against cancers with limited treatment options.
One of the primary cancers targeted by Enapotamab is Non-Small Cell Lung Cancer (NSCLC), where AXL overexpression plays a key role in tumor progression. Enapotamab’s ability to selectively bind to AXL on tumor cells allows it to inhibit cancer growth and metastasis, improving survival rates in patients who may not respond to standard chemotherapy or targeted treatments.
In melanoma, especially advanced or metastatic forms, Enapotamab shows promise by disrupting the pathways that drive tumor growth. Elevated AXL expression is common in melanoma, and Enapotamab’s targeted approach offers a novel therapeutic option for patients who have limited responses to existing treatments such as immune checkpoint inhibitors.
Enapotamab also has potential in treating head-and-neck cancers, which are often challenging due to limited effective therapies. By delivering cytotoxic agents directly to AXL-expressing cancer cells, Enapotamab offers a new method of overcoming resistance and improving treatment outcomes.
Ongoing clinical trials are exploring Enapotamab in combination with immune checkpoint inhibitors and standard chemotherapy, aiming to optimize dosing and identify patient subgroups that will benefit most. Despite challenges like tumor heterogeneity, Enapotamab’s clinical development underscores its transformative potential in precision cancer therapy.
4.) Advancing Research on Enapotamab: The Role of Biosimilars
What is a Biosimilar?
A biosimilar is a biologic product highly similar to an already-approved reference product, with no clinically meaningful differences in safety, purity, or potency. In research, biosimilars serve as cost-effective tools for studying mechanisms, testing hypotheses, and validating therapeutic strategies.
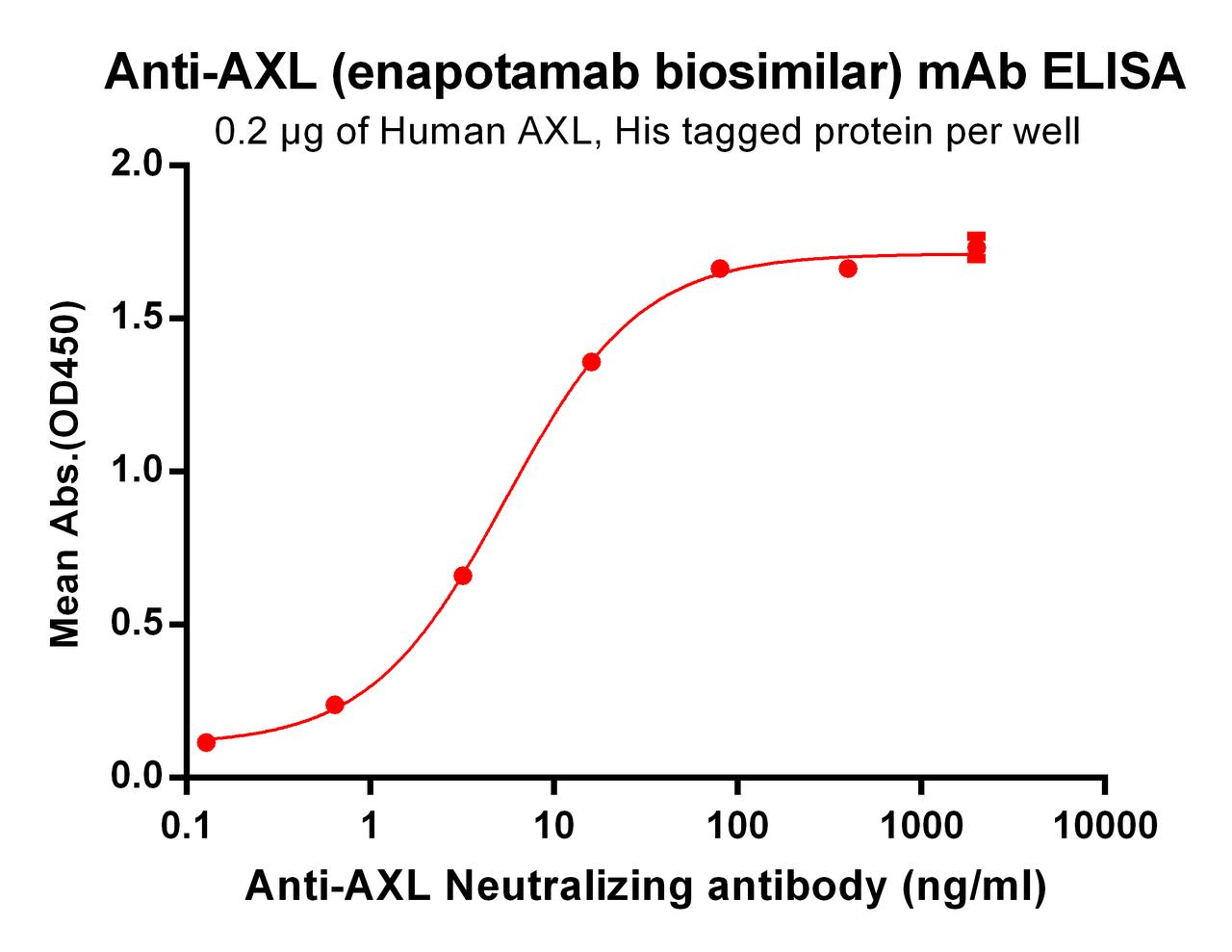
| Enapotamab (Anti-AXL) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | AXL |
| Reactivity: | Human |
How Enapotamab Biosimilar Compares to Enapotamab
Enapotamab biosimilars are designed to replicate the original ADC’s properties closely. While not identical, they maintain comparable efficacy and safety profiles, making them valuable for non-clinical research applications.
Benefits of Enapotamab Biosimilars in Research
- Cost-Effectiveness: Biosimilars reduce costs, enabling broader access to advanced therapeutic tools for preclinical studies.
- Scalability: Researchers can use biosimilars to explore Enapotamab’s mechanisms and optimize therapeutic strategies without relying on limited supplies of the original product.
- Innovation: By facilitating studies on ADC mechanisms, biosimilars support the development of next-generation therapeutics targeting AXL and similar pathways.
Research Use Only Disclaimer:
Enapotamab biosimilars are intended for research use only and are not approved for clinical or therapeutic applications. Researchers should use them solely for experimental and non-clinical purposes, adhering to ethical and regulatory standards.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Chris McNally, PhD
Chris McNally, PhD, has a strong foundation in Biomedical Science, completing a PhD scholarship in collaboration with Randox Laboratories and Ulster University. Chris has published extensively in prostate cancer research, focusing on biomarker discovery, cancer risk stratification, and molecular mechanisms such as hypoxia-induced regulation. He currently serves as a Business Development Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025




