Dupilumab: Transforming Immunotherapy and Skin Disease Management
What You Need to Know About Dupilumab
What is Dupilumab?
Dupilumab is a fully human monoclonal antibody designed to treat various chronic inflammatory diseases by inhibiting key cytokines involved in immune responses.
How does Dupilumab work?
It targets the interleukin-4 (IL-4) receptor α subunit, blocking IL-4 and IL-13 signaling pathways that drive inflammation.
What are the clinical applications of Dupilumab?
Approved for atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis (CRSwNP), and eosinophilic esophagitis (EoE), Dupilumab addresses several unmet medical needs.
Is Dupilumab safe?
Yes, Dupilumab has a well-established safety profile, with mild injection-site reactions and transient eosinophilia among the most common side effects.
1.) Understanding Dupilumab
Dupilumab has redefined the therapeutic landscape for chronic inflammatory diseases by offering targeted intervention at the molecular level. Developed through a collaboration between Regeneron and Sanofi, this monoclonal antibody was the first biologic therapy approved for moderate-to-severe atopic dermatitis in 2017. Its approval marked a major breakthrough, providing an effective treatment option for patients who had struggled with conventional therapies such as topical steroids, systemic immunosuppressants, and phototherapy. By addressing the underlying inflammatory pathways rather than merely alleviating symptoms, Dupilumab has set a new standard for long-term disease management.
Beyond its dermatological applications, Dupilumab’s versatility extends to respiratory and gastrointestinal conditions, particularly those driven by type 2 inflammation. Its dual inhibition of interleukin-4 (IL-4) and interleukin-13 (IL-13), two cytokines responsible for allergic inflammation, makes it a cornerstone therapy for asthma, chronic rhinosinusitis with nasal polyps (CRSwNP), and eosinophilic esophagitis (EoE). By blocking these key inflammatory pathways, Dupilumab helps reduce airway inflammation, nasal congestion, and eosinophilic infiltration, leading to significant improvements in lung function, reduced exacerbations, and better overall disease control.
Ongoing research continues to explore the potential of Dupilumab in additional indications, including food allergies, chronic obstructive pulmonary disease (COPD), and even other immune-mediated disorders. This broad-spectrum applicability highlights its transformative potential in immunotherapy. Furthermore, its favorable safety profile, combined with its ability to reduce reliance on systemic steroids and other immunosuppressants, has positioned Dupilumab as a preferred long-term treatment option. By improving disease control and enhancing patients' quality of life, Dupilumab continues to reshape the management of chronic inflammatory diseases.
2.) Mechanism of Action of Dupilumab
The mechanism of action of Dupilumab revolves around its ability to precisely modulate type 2 inflammation, a key driver of multiple chronic inflammatory conditions. It achieves this by targeting the IL-4 receptor α subunit, a shared component of the receptor complexes for interleukin-4 (IL-4) and interleukin-13 (IL-13). These cytokines play central roles in immune cell activation, IgE production, eosinophil recruitment, and mucus hypersecretion—all hallmarks of type 2 inflammatory diseases. By inhibiting their signaling, Dupilumab effectively reduces inflammation at both systemic and tissue-specific levels, offering a highly targeted therapeutic approach.
Upon administration, Dupilumab binds to the IL-4 receptor α subunit, preventing downstream signaling that would otherwise drive chronic inflammation. This blockade reduces immune cell activation and suppresses the release of pro-inflammatory mediators. Unlike broad-spectrum immunosuppressants, Dupilumab provides targeted immunomodulation without significantly compromising overall immune function. This selective mechanism minimizes the risk of opportunistic infections and other adverse effects commonly associated with traditional immunosuppressive therapies, making it a safer long-term option for patients with chronic inflammatory diseases.
Preclinical studies and clinical trials have demonstrated Dupilumab’s precision and efficacy across multiple disease states. In moderate-to-severe atopic dermatitis, it has been shown to reduce disease severity by over 75% in many cases, significantly improving skin clarity and reducing pruritus. In asthma, Dupilumab has been proven to lower exacerbation rates, enhance lung function, and decrease corticosteroid dependency. Its success in treating chronic rhinosinusitis with nasal polyps (CRSwNP) and eosinophilic esophagitis (EoE) further highlights its broad therapeutic potential. As a disease-modifying therapy, Dupilumab offers long-term benefits while avoiding the pitfalls of conventional treatments, redefining the management of type 2 inflammatory diseases.
3.) Clinical Applications of Dupilumab
Dupilumab has demonstrated efficacy across a broad spectrum of chronic inflammatory diseases, making it one of the most versatile biologic therapies available. Initially approved for moderate-to-severe atopic dermatitis, its indications have since expanded to include asthma, chronic rhinosinusitis with nasal polyps (CRSwNP), and eosinophilic esophagitis (EoE). These conditions share a common underlying mechanism of type 2 inflammation, which Dupilumab effectively suppresses by blocking IL-4 and IL-13 signaling, offering targeted immune modulation with long-lasting benefits.
In dermatology, Dupilumab has revolutionized the management of atopic dermatitis, providing rapid and sustained symptom relief for patients who previously relied on systemic corticosteroids or immunosuppressants. Clinical trials have shown that a significant proportion of patients achieve at least a 75% reduction in disease severity (EASI-75) after treatment initiation, with many experiencing near-complete disease control. This has led to profound improvements in quality of life, alleviating persistent itching, skin lesions, and sleep disturbances while reducing dependence on conventional therapies.
In respiratory medicine, Dupilumab has been a breakthrough therapy for patients with severe, corticosteroid-dependent asthma. Studies have demonstrated substantial reductions in exacerbations, improved lung function, and decreased reliance on systemic steroids, leading to better disease control. Similarly, in CRSwNP, Dupilumab has been shown to significantly reduce nasal congestion, shrink polyp size, and decrease the need for surgical interventions.
Ongoing research continues to explore Dupilumab’s role in other immune-mediated conditions, such as food allergies and chronic obstructive pulmonary disease (COPD). Its expanding range of indications suggests that it may further redefine treatment paradigms in immunology, offering safer and more effective alternatives to traditional therapies while improving long-term patient outcomes.
4.) Exploring Biosimilars for Dupilumab
What is a Biosimilar?
A biosimilar is a biologic product that matches the original reference product in terms of structure, efficacy, and safety. Biosimilars provide affordable alternatives, enabling broader access to advanced therapies and fostering innovation in medical research.
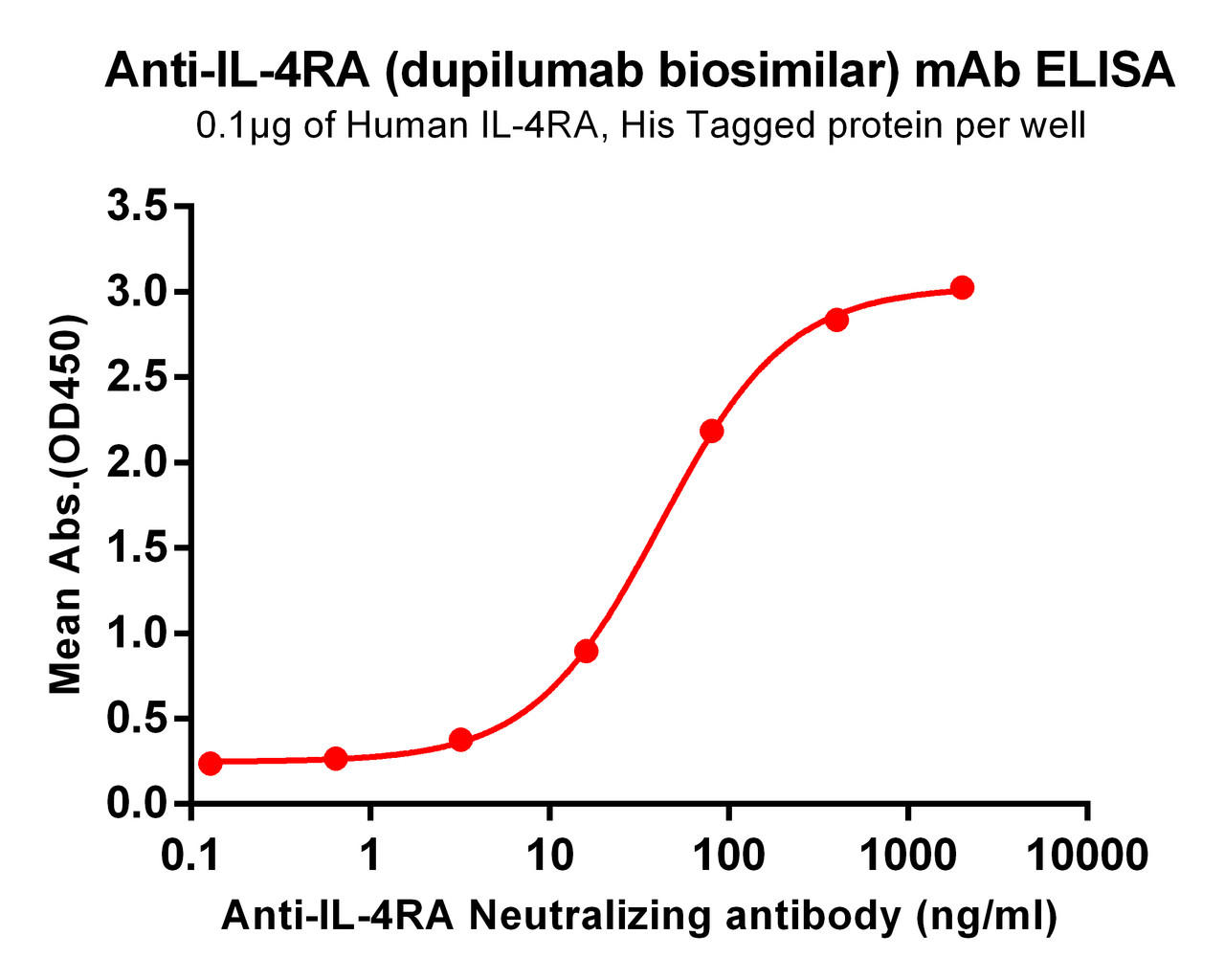
| Dupilumab (Anti-IL-4RA) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | IL-4RA |
| Reactivity: | Human |
How Does Dupilumab Biosimilar Compare?
Our Dupilumab biosimilar is designed to mirror the original in molecular function and therapeutic impact. While not intended for clinical use, it serves as a vital resource for preclinical and translational research.
Benefits of Dupilumab Biosimilar for Research
- Cost-Effectiveness: Reduces expenses associated with high-cost biologics in exploratory studies.
- High Quality: Maintains the structural and functional integrity of the reference product, ensuring reliable research outcomes.
- Accelerating Discovery: Supports the development of next-generation biologics and personalized therapies.
Note: The Dupilumab biosimilar is for research use only and is not intended for diagnostic or therapeutic applications.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By David Lee, PhD
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Indusatumab: Advancing Anti-CD47 Cancer Research with Biosimilar Innovations
Quick Facts About IndusatumabWhat is Indusatumab?Indusatumab is an experimental antibo …8th Feb 2025


