Denosumab: A Comprehensive Overview of Mechanism, Applications, and Research Advances
What You Need to Know About Denosumab
What is Denosumab?
Denosumab is a monoclonal antibody used primarily to treat osteoporosis and bone-related complications in cancer patients. It targets RANKL (Receptor Activator of Nuclear Factor Kappa-Β Ligand), inhibiting osteoclast formation and activity to prevent bone resorption.
What is the mechanism of action for Denosumab?
Denosumab works by binding to RANKL, a protein responsible for stimulating the formation and function of osteoclasts, which are cells that break down bone tissue. By inhibiting RANKL, Denosumab reduces osteoclast activity and bone resorption.
What are the clinical applications of Denosumab?
Denosumab is used to treat osteoporosis, prevent fractures in individuals with bone metastases from cancer, and manage other conditions associated with excessive bone resorption, such as giant cell tumor of the bone.
1.) Understanding Denosumab
Denosumab is a groundbreaking therapeutic agent primarily used for managing bone-related diseases, particularly those involving accelerated bone resorption. The drug functions by targeting RANKL (Receptor Activator of Nuclear Factor Kappa-Β Ligand), a critical molecule in the regulation of bone metabolism. RANKL plays an essential role in the activation and differentiation of osteoclasts, the cells responsible for breaking down bone tissue. In conditions like osteoporosis, where the balance between bone resorption and formation is disrupted, osteoclasts become overactive, leading to excessive bone loss and an increased risk of fractures.
Developed as a monoclonal antibody, Denosumab selectively binds to RANKL and prevents its interaction with RANK, the receptor on osteoclasts. This inhibition suppresses osteoclast activation, reducing bone degradation. Unlike bisphosphonates, which also aim to reduce bone resorption but work through a different pathway by binding to bone minerals, Denosumab offers a more targeted approach. Its ability to block RANKL makes it distinct in its therapeutic potential, particularly for patients with diseases like osteoporosis and metastatic bone cancer.
Denosumab’s efficacy has made it a preferred option for patients at high risk of fractures due to weakened bones. It has shown significant clinical benefits in both men and women suffering from osteoporosis, especially those with postmenopausal osteoporosis. It is increasingly being explored for use in other bone-related conditions, such as giant cell tumors and bone metastasis in cancer patients. Ongoing research is further investigating Denosumab’s safety profile, long-term effects, and potential applications for broader clinical use.
2.) Mechanism of Action of Denosumab
Denosumab’s mechanism of action revolves around its ability to target RANKL, a key signaling molecule involved in bone resorption. Bone remodeling is a dynamic process in which old bone tissue is broken down by osteoclasts and replaced by new bone formed by osteoblasts. This process is crucial for maintaining bone density and strength. However, when osteoclast activity becomes excessive, as seen in diseases like osteoporosis, bone breakdown outpaces bone formation, leading to weakened bones and an increased risk of fractures.
RANKL plays a central role in the regulation of osteoclast activity by binding to its receptor, RANK, on osteoclast precursor cells and mature osteoclasts. This binding initiates a cascade of signaling events that promote the formation, activation, and survival of osteoclasts. Denosumab, a fully human monoclonal antibody, works by specifically binding to RANKL, preventing it from interacting with RANK. As a result, the activation and function of osteoclasts are inhibited, leading to reduced bone resorption.
The drug’s selective inhibition of RANKL makes it an effective treatment for conditions characterized by excessive bone resorption, such as osteoporosis, metastatic bone disease, and bone loss associated with certain cancer treatments. Denosumab’s effects are reversible, unlike bisphosphonates, which are incorporated into the bone matrix and persist for extended periods. This feature provides greater flexibility in treatment, allowing for adjustments in therapy based on patient needs. Moreover, Denosumab’s rapid onset of action offers a valuable advantage, particularly in acute bone loss situations.
3.) Clinical Applications of Denosumab
Denosumab has demonstrated substantial clinical benefits in treating various bone-related conditions, with its most well-established application being in the management of osteoporosis. Osteoporosis is a condition that leads to decreased bone mass and density, making bones fragile and more susceptible to fractures. Denosumab has proven effective in significantly increasing bone mineral density (BMD) in postmenopausal women and men at risk for osteoporotic fractures. Clinical studies have shown that Denosumab reduces the risk of vertebral, hip, and nonvertebral fractures, making it a key therapeutic option for osteoporosis management.
In addition to its role in osteoporosis, Denosumab is also used to prevent skeletal-related events (SREs) in patients with bone metastases from cancer. Metastatic bone disease can lead to debilitating complications, such as fractures, spinal cord compression, and the need for radiation or surgery. Denosumab has been shown to reduce the incidence of these events, improving the quality of life for cancer patients with bone metastases. By inhibiting bone resorption, Denosumab helps maintain bone integrity and prevent the severe consequences of metastatic bone involvement.
Emerging research has also explored Denosumab’s potential in treating other bone diseases, including giant cell tumors of the bone, a rare and aggressive tumor type. Preliminary studies suggest that Denosumab may be effective in shrinking these tumors and improving patient outcomes, especially for those who have not responded well to other treatments. Furthermore, Denosumab is being investigated as a preventive treatment for bone loss in cancer patients undergoing therapies like chemotherapy and hormone treatments, which can lead to bone demineralization. As ongoing research continues, Denosumab’s therapeutic applications in various bone diseases are expanding, offering hope for improved treatment outcomes and quality of life for patients with bone-related conditions.
4.) Exploring the Biosimilar Product
Biosimilars are biological products that are highly similar to an already-approved reference product but are not identical. Denosumab biosimilars are designed to mimic the therapeutic effects of the original Denosumab, providing an alternative for research applications. They are developed to be as safe and effective as the original drug, offering researchers a cost-effective way to explore Denosumab’s biological effects without the higher price of the branded version.
What is a Biosimilar?
A biosimilar is a biologic medical product that is highly similar to an existing FDA-approved reference product. While not identical, a biosimilar matches the original product in terms of structure, function, and clinical efficacy, making it a valuable tool for research and treatment development.
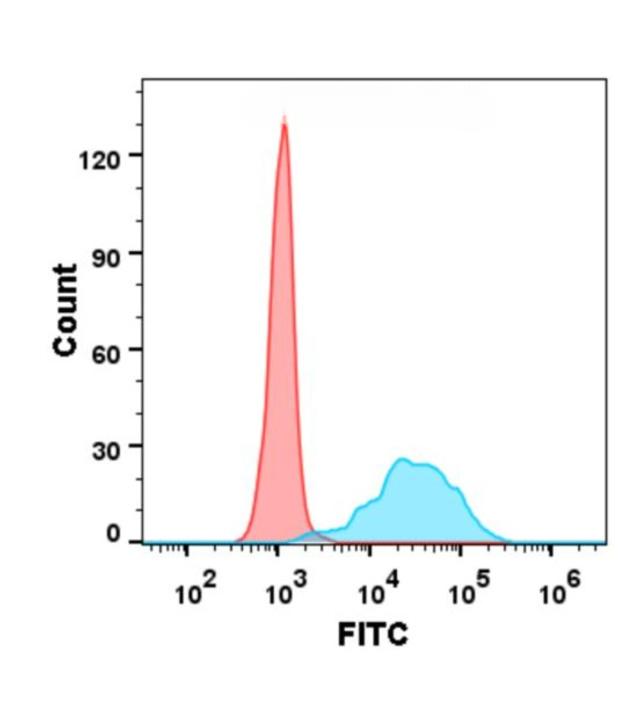
| Denosumab (Anti-TNFSF11) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | TNFSF11 |
| Reactivity: | Human |
Comparison
Denosumab biosimilars share the same mechanism of action as the original Denosumab, targeting RANKL to prevent osteoclast formation. While there may be minor differences in the manufacturing process, biosimilars are subject to rigorous testing to ensure they provide the same clinical benefits as their reference products.
Benefits for Research
Denosumab biosimilars provide a significant benefit to researchers by offering a more affordable option for studying bone resorption, osteoporosis, and cancer metastasis. They allow for large-scale experiments and studies without the financial burden of the branded version, facilitating more extensive research.
Research Use Only Disclaimer:
Denosumab biosimilars are primarily intended for research use only and should not be used for clinical treatment outside of approved studies. They serve as an important tool in advancing our understanding of bone-related diseases and the mechanisms behind therapeutic interventions.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Chris McNally, PhD
Chris McNally, PhD, has a strong foundation in Biomedical Science, completing a PhD scholarship in collaboration with Randox Laboratories and Ulster University. Chris has published extensively in prostate cancer research, focusing on biomarker discovery, cancer risk stratification, and molecular mechanisms such as hypoxia-induced regulation. He currently serves as a Business Development Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



