Crizanlizumab: A Comprehensive Overview of Its Role in Sickle Cell Disease and Ongoing Research
Quick Facts About Crizanlizumab
What is Crizanlizumab?
Crizanlizumab is a monoclonal antibody used to treat sickle cell disease by targeting P-selectin, a protein involved in inflammation and blood cell adhesion.
What is the mechanism of action for Crizanlizumab?
Crizanlizumab works by inhibiting P-selectin, reducing sickling of red blood cells, and decreasing the frequency of vaso-occlusive crises in patients with sickle cell disease.
What are the clinical applications of Crizanlizumab?
Crizanlizumab is primarily used in sickle cell disease treatment, particularly for patients experiencing frequent pain episodes (vaso-occlusive crises).
Is Crizanlizumab FDA-approved?
Yes, Crizanlizumab has received FDA approval for treating sickle cell disease in adults and pediatric patients aged 16 and older.
1.) Understanding Crizanlizumab
Crizanlizumab is a groundbreaking therapy specifically designed to treat sickle cell disease (SCD), a genetic blood disorder that causes red blood cells to adopt a sickle-like shape. This abnormal cell shape leads to reduced oxygen transport, blocked blood flow, and painful episodes known as vaso-occlusive crises. These episodes are caused when the sickle cells adhere to the walls of blood vessels, obstructing the flow of blood and leading to tissue damage and severe pain. Traditional treatments for sickle cell disease, such as hydroxyurea, have limitations, particularly in patients who continue to experience frequent crises or suffer from significant side effects. Crizanlizumab offers a unique mechanism of action by targeting and blocking P-selectin, a protein expressed on endothelial cells and platelets. P-selectin plays a pivotal role in the adhesion of sickle cells to blood vessel walls, which is a key step in the development of vaso-occlusive crises. By inhibiting P-selectin, Crizanlizumab reduces this adhesion, helping to prevent the formation of blockages that cause pain and complications.
Crizanlizumab provides a novel approach to managing SCD, focusing on addressing the root cause of vaso-occlusion rather than just alleviating symptoms. This drug has shown significant promise in clinical trials, demonstrating the ability to reduce the frequency of crises and improving the quality of life for many patients. The drug's potential is not limited to SCD alone, as ongoing research is exploring its use in other inflammatory conditions. As studies continue, Crizanlizumab’s role in treating sickle cell disease and other disorders expands, signaling its importance in future medical treatments.
2.) Mechanism of Action of Crizanlizumab
Crizanlizumab’s mechanism of action centers on its ability to block the function of P-selectin, a critical protein involved in the adhesion process between sickle cells and blood vessel walls. Sickle-shaped red blood cells are less flexible than normal cells, and they tend to become sticky, which leads to clumping and the formation of blockages in small blood vessels, causing vaso-occlusive crises. P-selectin, which is present on both the endothelial cells lining the blood vessels and platelets, facilitates this adhesion, thereby contributing to the blockage of blood flow. By binding to P-selectin, Crizanlizumab prevents its interaction with other molecules, such as integrins, which are responsible for binding the sickle cells to the blood vessel walls. This action effectively reduces the frequency and severity of vaso-occlusive crises in patients with sickle cell disease.
Unlike other treatments that target the sickle cells themselves, Crizanlizumab works by addressing the inflammatory and adhesive processes that drive the complications of the disease. The drug does not alter the shape of sickle cells, but rather works to reduce their tendency to cluster together and cause blockages. Crizanlizumab's targeted action on P-selectin helps improve blood flow, decreasing the painful episodes and the risk of organ damage that often occur as a result of sickle cell-related complications. Additionally, ongoing studies are exploring the potential for Crizanlizumab to prevent other serious issues related to sickle cell disease, such as stroke and damage to vital organs, further expanding the therapeutic possibilities of the drug. As research progresses, Crizanlizumab could become an essential part of the management of sickle cell disease, as well as other inflammatory conditions where P-selectin plays a role.
3.) Clinical Applications of Crizanlizumab
Crizanlizumab is primarily indicated for the treatment of sickle cell disease (SCD), where it has demonstrated substantial effectiveness in reducing the frequency of vaso-occlusive crises, which are debilitating and often lead to long-term complications. In a key Phase 3 clinical trial, the SUSTAIN study, Crizanlizumab significantly reduced the occurrence of these painful episodes compared to a placebo, offering considerable relief to patients who suffer from recurrent crises. This makes Crizanlizumab an important treatment option for individuals with sickle cell disease who experience frequent or severe episodes that cannot be adequately controlled with other therapies.
Crizanlizumab’s impact on sickle cell disease is particularly noteworthy because it represents a novel approach in managing the disease. Traditional treatments such as hydroxyurea or regular blood transfusions are effective for some patients, but they may not fully address the underlying mechanisms of vaso-occlusion. By targeting P-selectin, Crizanlizumab tackles the root cause of these crises, reducing cell adhesion and improving blood flow. This makes it a valuable addition to the treatment arsenal for SCD.
In addition to its primary use in sickle cell disease, Crizanlizumab is also being studied for its potential to prevent other complications associated with SCD, such as strokes and organ damage. These complications are common in patients with SCD and can significantly affect quality of life and life expectancy. Emerging research suggests that Crizanlizumab could play a role in reducing the incidence of these severe complications, although further studies are needed to confirm its efficacy in these areas. The drug is currently FDA-approved for adults and pediatric patients aged 16 and older, and ongoing clinical trials are exploring its use in younger children and assessing its long-term safety and effectiveness.
Furthermore, Crizanlizumab is being evaluated in combination with other therapies to determine whether it can offer synergistic benefits. Researchers are investigating whether combining Crizanlizumab with other drugs can enhance its effects and provide even more effective management of sickle cell disease. As the body of research surrounding Crizanlizumab grows, its clinical applications are expected to expand, potentially offering new hope for patients with SCD and other conditions associated with P-selectin.
4.) Exploring Crizanlizumab Biosimilars
What is a Biosimilar?
A biosimilar is a biologic product that is highly similar to an already approved reference product, with no clinically meaningful differences in terms of safety, purity, and potency. Biosimilars provide researchers and healthcare providers with affordable alternatives, making treatments more accessible while enabling further clinical exploration and innovation.
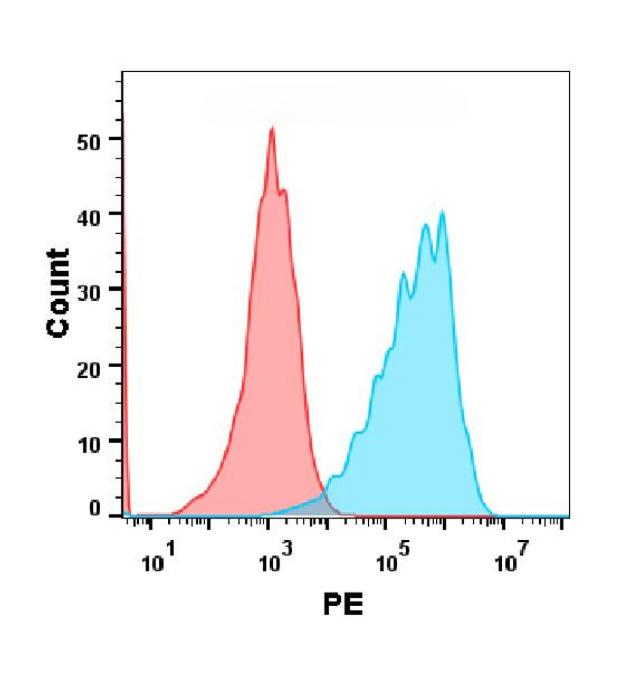
| Crizanlizumab (Anti-SELP) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | SELP |
| Reactivity: | Human |
Crizanlizumab Biosimilar in Research
Emerging biosimilars of Crizanlizumab offer valuable research opportunities, especially in the context of clinical trials and studies that seek to understand the broader applications of P-selectin inhibition. These biosimilars are particularly useful for comparative studies and for exploring how minor variations in production processes can impact efficacy and patient outcomes.
By offering a cost-effective alternative, Crizanlizumab biosimilars can facilitate wider access to treatment in clinical research settings. Their development allows for large-scale studies that could provide deeper insights into the drug’s mechanism and its broader therapeutic applications. Notably, biosimilars are typically used for research purposes only and are not intended for direct patient use until they receive appropriate regulatory approval.
These biosimilars not only offer an opportunity to understand the nuances of Crizanlizumab’s treatment profile but also contribute to the global understanding of biologic therapies in rare and chronic diseases like sickle cell disease.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Chris McNally, PhD
Chris McNally, PhD, has a strong foundation in Biomedical Science, completing a PhD scholarship in collaboration with Randox Laboratories and Ulster University. Chris has published extensively in prostate cancer research, focusing on biomarker discovery, cancer risk stratification, and molecular mechanisms such as hypoxia-induced regulation. He currently serves as a Business Development Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Crizanlizumab: A Comprehensive Overview of Its Role in Sickle Cell Disease and Ongoing Research
Quick Facts About CrizanlizumabWhat is Crizanlizumab?Crizanlizumab is a monoclonal ant …6th Feb 2025


