Cevostamab: Unveiling the Role of Anti-CD47 in Cancer Research
Quick Info Section: Key Facts About Cevostamab
What is Cevostamab?
Cevostamab is an investigational monoclonal antibody targeting CD47, a cell surface protein that helps cancer cells evade the immune system. By blocking CD47, Cevostamab enhances immune response and aids in cancer cell destruction.
What is the mechanism of action of Cevostamab?
Cevostamab works by inhibiting CD47, a "don't eat me" signal that prevents macrophages from attacking cancer cells. This action promotes the recognition and elimination of tumor cells by the immune system.
What are the clinical applications of Cevostamab?
Cevostamab has shown promise in treating various cancers, particularly multiple myeloma, by enhancing anti-tumor immunity. Ongoing clinical trials are exploring its potential in other hematologic malignancies and solid tumors.
1.) Understanding Cevostamab
Cevostamab is a revolutionary monoclonal antibody that targets CD47, a cell surface protein that plays a critical role in immune evasion by cancer cells. CD47 serves as a “don’t eat me” signal to the immune system, specifically inhibiting macrophages and other immune cells from attacking and engulfing the cancer cells. By blocking this protective signal, Cevostamab effectively encourages the body’s immune system to recognize and destroy cancerous cells. This unique approach makes Cevostamab a promising therapeutic agent in oncology, especially for cancers that have developed resistance to conventional treatments.
The underlying principle behind Cevostamab’s action lies in its ability to remove the immunosuppressive shield that cancer cells utilize to escape immune surveillance. By binding to CD47, Cevostamab disrupts this communication between cancer cells and the immune system, facilitating the recognition and elimination of tumors. As a result, it enhances the body’s natural immune response, making it a key player in the growing field of immuno-oncology.
Cevostamab is being actively studied in clinical trials, particularly for its potential in treating hematologic cancers like multiple myeloma. These trials aim to assess not only the safety and efficacy of the drug but also its ability to work in combination with other cancer therapies, such as monoclonal antibodies or immune checkpoint inhibitors. This combination strategy holds the promise of improving treatment outcomes for patients with cancers that are difficult to treat with traditional therapies, offering a new hope for those with limited options.
2.) Mechanism of Action of Cevostamab
The core mechanism of action of Cevostamab revolves around its ability to block the CD47 receptor, a protein found on the surface of many cancer cells. CD47 plays an essential role in helping tumor cells avoid detection by the immune system. It does so by signaling to macrophages, a type of immune cell, that the cancer cell should not be eaten or attacked. This immune evasion mechanism is one of the reasons why cancer cells can proliferate unchecked in the body.
Cevostamab works by binding directly to CD47, preventing its interaction with its receptor, SIRPα, on macrophages and other immune cells. This blockage of the "don’t eat me" signal removes the protection for cancer cells, allowing immune cells, particularly macrophages, to recognize and phagocytose the tumor cells. By enhancing the body’s immune surveillance, Cevostamab encourages the immune system to more effectively target and destroy cancerous cells, offering an alternative to traditional therapies like chemotherapy and radiation that often have significant side effects.
Additionally, Cevostamab’s immuno-oncology approach has shown potential in amplifying the effects of other cancer therapies. When used in combination with monoclonal antibodies or immune checkpoint inhibitors, Cevostamab can synergistically boost the immune response, further enhancing the efficacy of cancer treatment. This approach provides a promising avenue for patients with cancers that have been resistant to other treatments, such as hematologic malignancies like multiple myeloma. As more research is conducted, it is likely that Cevostamab will become an integral part of immunotherapy regimens aimed at improving patient outcomes.
3.) Clinical Applications of Cevostamab
Cevostamab is currently being explored in clinical trials for its potential to treat a variety of cancers, particularly hematologic malignancies like multiple myeloma. Early-phase trials have demonstrated that Cevostamab can enhance the immune system's ability to target and eliminate cancerous cells, even in patients who have shown resistance to conventional therapies such as chemotherapy or stem cell transplants. This capability is especially important in cancers like multiple myeloma, which are known for their ability to relapse or become resistant to treatment over time.
The promising results of Cevostamab in preclinical studies have led to its investigation in combination with other immunotherapies. Researchers are examining how Cevostamab interacts with monoclonal antibodies, immune checkpoint inhibitors, and other immune-based treatments. These combination therapies are designed to further stimulate the immune system, creating a multi-pronged attack on the tumor. Early findings suggest that Cevostamab could improve the overall efficacy of these treatments, leading to better patient outcomes, especially in those with cancers that have limited treatment options.
While the drug's use in solid tumors is still in the experimental stage, the therapeutic potential of Cevostamab is undeniable. Ongoing clinical trials are exploring its role in a range of malignancies, with researchers hopeful that its ability to enhance immune responses will extend beyond hematologic cancers. As studies continue to progress, Cevostamab holds the potential to revolutionize cancer treatment by offering a novel, immune-focused therapy that could improve survival rates and quality of life for patients battling cancer.
4.) Advancing Research on Cevostamab with Biosimilars
While Cevostamab holds great promise as an anti-cancer agent, its biosimilar—Cevostamab biosimilar without anti-CD3 portion—provides a crucial tool for ongoing research. This biosimilar retains the essential therapeutic properties of Cevostamab but lacks the anti-CD3 domain, offering researchers a unique perspective on how targeting CD47 alone can impact immune response in cancer treatment.
The absence of the anti-CD3 portion does not diminish the potential of this biosimilar. On the contrary, it enables more precise investigations into how CD47 blockade alone affects the immune system, facilitating a deeper understanding of its role in immune evasion by tumors. Researchers can study this version without the added complexity of the anti-CD3 component, which helps clarify the specific contributions of CD47 inhibition.
What is a Biosimilar?
A biosimilar is a biologic product that is highly similar to an already approved reference product, such as Cevostamab. While biosimilars are not identical to their reference drug, they have no clinically meaningful differences in terms of safety, purity, or potency. In research, biosimilars provide valuable tools for scientists to explore different aspects of drug action and optimize therapeutic strategies, often at a lower cost than the original branded drug.
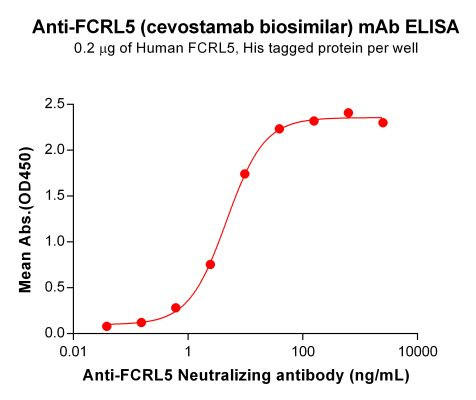
| Cevostamab (Anti-FCRL5 no CD3) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | FCRL5 |
| Reactivity: | Human |
Comparison: Cevostamab vs. Cevostamab Biosimilar
The Cevostamab biosimilar without the anti-CD3 portion is structurally similar to the original Cevostamab but differs in its composition, lacking the anti-CD3 component that would otherwise target T-cells. This key difference allows for a more focused investigation into the role of CD47 blockade in the immune response.
While the original Cevostamab is being explored in clinical applications, the biosimilar offers a promising tool for pre-clinical studies and laboratory research. It provides a platform for assessing the efficacy and potential side effects of CD47 blockade without the added complexity of interacting with T-cell mechanisms.
Benefits of the Biosimilar in Research
The Cevostamab biosimilar without anti-CD3 portion is invaluable in research, as it allows for a more specific focus on how CD47 inhibition alone affects tumor progression and immune system interactions. This specialized focus makes the biosimilar an essential tool for advancing our understanding of immune-oncology treatments.
Moreover, the lower cost and availability of the biosimilar make it an attractive option for academic and industry researchers looking to explore novel therapeutic approaches without the financial burden of the original drug.
Research Use Only Disclaimer:
Cevostamab biosimilar without anti-CD3 portion is for research use only and is not approved for clinical use.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Miren Ruiz de Eguilaz, PhD
Miren Ruiz de Eguilaz, PhD, has an extensive academic background, earning a BSc in Biology from UPV/EHU, an MSc in Biotechnology from the University of Oviedo, and a PhD in Chemistry from Dublin City University (DCU). Miren’s expertise lies in biosensor technology and bacterial diagnostics. She currently serves as a Product Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



