Cetuximab: A Revolutionary Drug in Cancer Therapy
Quick Facts About Cetuximab
What is Cetuximab?
Cetuximab is a monoclonal antibody targeting the epidermal growth factor receptor (EGFR), widely used in cancer therapy.
How does Cetuximab work?
Cetuximab binds to EGFR on cancer cells, inhibiting cell proliferation and inducing apoptosis, making it a powerful tool in oncology.
What are the clinical uses of Cetuximab?
Is Cetuximab safe?
Cetuximab is generally well-tolerated, but common side effects include skin rash and infusion reactions.
1.) Understanding Cetuximab
Cetuximab, a chimeric monoclonal antibody, has become a cornerstone in the treatment of cancers associated with epidermal growth factor receptor (EGFR) dysregulation. FDA-approved for specific malignancies such as metastatic colorectal cancer (mCRC) and head and neck squamous cell carcinoma (HNSCC), Cetuximab works by targeting EGFR, a receptor commonly overexpressed in many cancers. By binding to EGFR, it inhibits downstream signaling pathways that drive tumor cell proliferation, angiogenesis, and survival, thereby disrupting tumor growth and progression. This precision-based approach has solidified Cetuximab's place in oncology as a vital tool in combating aggressive cancers.
Emerging research highlights Cetuximab's significant impact on extending survival rates, particularly when used in combination with chemotherapy or radiation therapy. For instance, in mCRC, Cetuximab has demonstrated improved overall survival and progression-free survival when used with FOLFIRI or FOLFOX regimens. In HNSCC, its combination with radiation therapy has shown to enhance locoregional control, leading to better clinical outcomes. Beyond these indications, Cetuximab is also being investigated in the context of combination therapies to further enhance its therapeutic efficacy.
Recent studies are exploring the synergy between Cetuximab and immunotherapies, such as checkpoint inhibitors, aiming to bolster anti-tumor immune responses. Additionally, researchers are evaluating its integration with novel targeted agents to address resistance mechanisms that often limit its efficacy. Personalized medicine strategies leveraging molecular profiling are also underway to identify biomarkers that predict response to Cetuximab, paving the way for more tailored treatment approaches. These advancements position Cetuximab as a pivotal agent in the evolving landscape of oncology therapeutics.
2.) Mechanism of Action of Cetuximab
Cetuximab exerts its therapeutic effects by specifically targeting the extracellular domain of the epidermal growth factor receptor (EGFR), a transmembrane protein pivotal to cellular signaling pathways regulating proliferation, survival, differentiation, and angiogenesis. EGFR is frequently overexpressed or mutated in numerous cancers, leading to aberrant activation of signaling cascades that drive uncontrolled cell division, tumor progression, and metastasis. Cetuximab's ability to inhibit EGFR activity addresses these pathological processes directly.
By binding competitively to the extracellular domain of EGFR, Cetuximab prevents the receptor's activation by its natural ligands, such as EGF and TGF-α. This blockade halts downstream signaling through critical pathways, including the PI3K/AKT and RAS/RAF/MEK cascades. Consequently, Cetuximab induces several key anti-cancer effects:
1. Reduced Cell Proliferation: By interrupting growth-promoting signals, Cetuximab effectively curbs tumor expansion.
2. Induction of Apoptosis: The inhibition of survival pathways promotes programmed cell death in cancer cells that are dependent on EGFR signaling.
3. Enhanced Immune Response: Cetuximab also mediates antibody-dependent cellular cytotoxicity (ADCC), a process that recruits immune effector cells to target and destroy EGFR-expressing cancer cells.
Recent advancements underscore Cetuximab’s potential in overcoming resistance mechanisms, a critical challenge in targeted therapy. For instance, combining Cetuximab with other novel agents, such as small molecule inhibitors or immune checkpoint inhibitors, has shown promise in re-sensitizing resistant tumors. This combination strategy not only amplifies its therapeutic efficacy but also broadens its application across diverse cancer types. As a result, Cetuximab continues to hold a central role in the rapidly advancing field of precision oncology.
3.) Clinical Applications of Cetuximab
Cetuximab has proven to be a transformative treatment for cancers where EGFR dysregulation plays a pivotal role. Its primary FDA-approved indications include metastatic colorectal cancer (mCRC) and head and neck squamous cell carcinoma (HNSCC). In these malignancies, Cetuximab not only improves clinical outcomes but also serves as a cornerstone in multimodal treatment strategies.
For mCRC, Cetuximab is particularly effective in patients with wild-type KRAS or NRAS genes, as mutations in these pathways render the treatment ineffective. It is commonly administered in combination with irinotecan-based chemotherapy or as a single-agent therapy in patients who cannot tolerate conventional chemotherapeutics. Studies have demonstrated significant improvements in progression-free and overall survival in these populations, highlighting the drug's targeted efficacy.
In HNSCC, Cetuximab is invaluable for enhancing the therapeutic impact of radiation therapy, particularly in cases where surgery is not an option. It sensitizes tumors to radiation, improving local control and overall survival in both locoregionally advanced and recurrent/metastatic settings. Its utility in non-surgical management makes it a key option for improving patient quality of life.
Emerging applications are expanding Cetuximab’s role in oncology. Combination therapies are a particularly promising avenue, with ongoing clinical trials evaluating its synergistic effects when paired with immunotherapies, such as immune checkpoint inhibitors. Additionally, research is exploring its efficacy in other EGFR-dependent malignancies, including non-small cell lung cancer (NSCLC) and pancreatic cancer. These studies suggest Cetuximab could serve as a foundational agent in novel therapeutic regimens, particularly in precision oncology and combination approaches tailored to tumor biology.
4.) Exploring Biosimilars for Cetuximab
What is a Biosimilar?
Biosimilars are biologic medical products that are highly similar to an already approved reference product, such as Cetuximab. They match the reference product in terms of safety, efficacy, and quality but often offer cost advantages, making them invaluable for research and clinical applications.
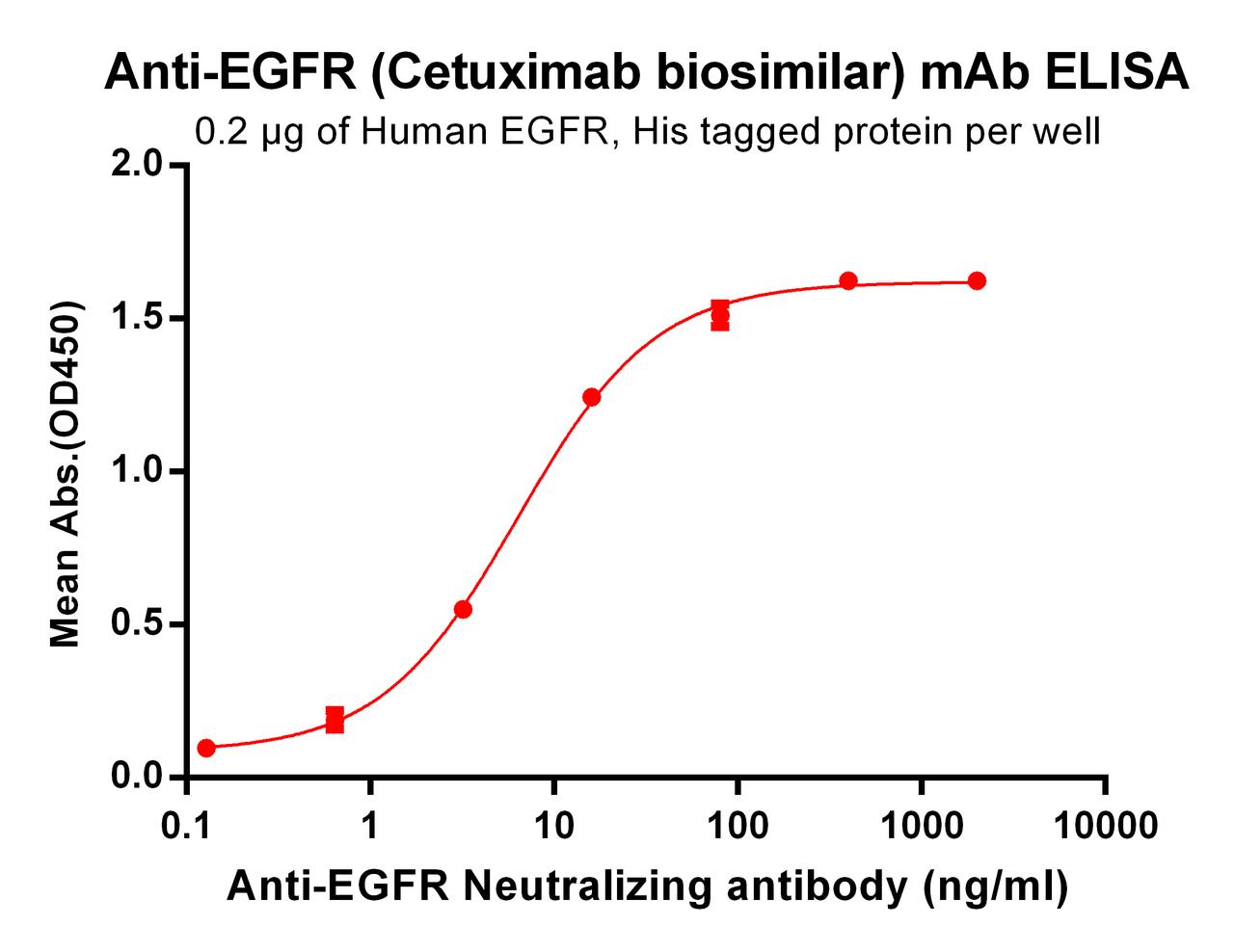
| Cetuximab (Anti-EGFR) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | EGFR |
| Reactivity: | Human |
How Cetuximab Biosimilar Compares to Cetuximab
The Cetuximab biosimilar mirrors the reference drug’s structure and function. While the original Cetuximab focuses on therapeutic use, the biosimilar is often employed for research purposes, enabling scientists to:
1. Conduct preclinical trials more cost-effectively.
2. Investigate EGFR-targeted therapies in a controlled setting.
3. Study resistance mechanisms and potential combination therapies.
Benefits of Cetuximab Biosimilars
- Accessibility: Biosimilars lower the cost barrier for advanced research.
- Consistency: They provide reproducible results, ensuring reliability in experimental setups.
- Innovation Catalyst: By reducing expenses, biosimilars enable broader exploration of novel therapeutic combinations.
Note:
Cetuximab biosimilars are intended for research use only and are not approved for direct therapeutic applications.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By David Lee, PhD
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Ivuxolimab: Redefining Cancer Immunotherapy and Research
Quick Facts About IvuxolimabWhat is ivuxolimab?Ivuxolimab is a monoclonal antibody tar …1st Feb 2025


