Carlumab: Exploring Its Mechanism, Clinical Potential, and Research Biosimilars
Quick Facts About Carlumab
What is Carlumab?
What is the mechanism of action of Carlumab?
What are the clinical applications of Carlumab?
1.) Understanding Carlumab
Carlumab is a fully human monoclonal antibody engineered to neutralize CCL2 (monocyte chemoattractant protein-1 or MCP-1), a key chemokine involved in inflammatory responses and tumor microenvironments. CCL2 plays a critical role in the recruitment and activation of monocytes, macrophages, and other immune cells, driving chronic inflammation and facilitating tumor progression by supporting angiogenesis, immune evasion, and metastasis. Given these roles, targeting CCL2 with Carlumab presents a potential strategy for modulating disease pathology in cancer, fibrosis, and autoimmune disorders.
Developed initially for oncology applications, Carlumab was expected to disrupt the tumor microenvironment by depleting tumor-associated macrophages (TAMs) and reducing cancer cell migration. However, early clinical trials produced mixed outcomes, as compensatory mechanisms led to increased CCL2 production, which may have limited the drug’s long-term efficacy. Despite these setbacks, Carlumab remains a subject of interest in research, particularly in preclinical studies exploring alternative administration strategies, optimized dosing regimens, and combination therapies that enhance its effectiveness.
Beyond oncology, Carlumab has been investigated in inflammatory conditions such as rheumatoid arthritis and idiopathic pulmonary fibrosis, given CCL2’s role in monocyte recruitment to inflamed tissues. Research continues to focus on refining CCL2-targeting approaches, integrating Carlumab into broader immunomodulatory treatment strategies, and evaluating its potential synergistic effects with other therapies, such as checkpoint inhibitors or fibrosis-targeting drugs. These ongoing studies are critical to fully understanding Carlumab’s therapeutic scope and optimizing its clinical utility.
2.) Mechanism of Action of Carlumab
Carlumab functions by selectively binding to CCL2, effectively preventing its interaction with its primary receptors, CCR2 and CCR4. This inhibition disrupts a key signaling axis responsible for monocyte and macrophage recruitment, which plays a significant role in various inflammatory and oncogenic processes. By blocking CCL2-CCR2 interactions, Carlumab aims to reduce the infiltration of monocytes and macrophages into diseased tissues, thereby mitigating inflammation, fibrosis, and tumor progression.
The CCL2-CCR2 pathway is implicated in multiple pathological conditions, including metastatic cancers, autoimmune disorders, and chronic inflammatory diseases such as pulmonary fibrosis and atherosclerosis. In the tumor microenvironment, CCL2-mediated recruitment of monocytes contributes to the formation of TAMs, which promote tumor growth by suppressing anti-tumor immune responses and enhancing angiogenesis. By inhibiting this process, Carlumab was initially expected to hinder tumor progression and improve patient outcomes. However, clinical trials revealed an unexpected compensatory increase in circulating CCL2 levels, which diminished the drug’s efficacy when used alone.
To address these challenges, researchers are exploring combination approaches that integrate Carlumab with other immunotherapies. For example, Carlumab has been proposed as a complementary agent to checkpoint inhibitors, which reactivates the immune system’s ability to attack tumors. Additionally, dosing optimizations and novel drug delivery systems are being investigated to enhance its pharmacokinetic profile and sustain CCL2 suppression more effectively. These refinements may improve Carlumab’s therapeutic potential across a broader range of inflammatory and oncologic conditions.
3.) Clinical Applications of Carlumab
Carlumab has undergone clinical evaluation in multiple therapeutic areas, particularly in oncology and inflammatory diseases. Initial studies highlighted its potential to modulate immune cell infiltration, reduce tumor-associated inflammation, and slow disease progression in metastatic cancers. However, despite promising preclinical data, clinical trials faced significant hurdles. The most notable challenge was the transient nature of CCL2 suppression, as the body’s compensatory feedback mechanisms led to increased CCL2 production, thereby limiting Carlumab’s sustained efficacy.
In cancer research, Carlumab was primarily tested in advanced solid tumors, including prostate and lung cancers, where CCL2 is known to contribute to tumor growth and metastasis. However, due to compensatory upregulation of CCL2, its use as a monotherapy showed limited benefit. Consequently, research has shifted towards evaluating Carlumab in combination with other immunotherapies, such as PD-1/PD-L1 inhibitors, to enhance its anti-tumor effects. Ongoing trials aim to determine whether Carlumab can improve immune infiltration and response rates when paired with checkpoint blockade strategies.
Beyond oncology, Carlumab has been investigated for its potential in treating chronic inflammatory diseases, including idiopathic pulmonary fibrosis (IPF) and rheumatoid arthritis. Since CCL2 is a key driver of monocyte recruitment in fibrotic and autoimmune conditions, inhibiting this pathway may help alleviate disease progression. While early clinical studies did not meet their primary endpoints, they provided valuable insights into the role of CCL2 in these diseases, paving the way for next-generation therapeutic strategies.
Despite setbacks in clinical trials, Carlumab remains a valuable research tool for understanding CCL2-mediated pathologies. Current studies are focused on refining patient selection criteria, optimizing combination approaches, and developing novel formulations that sustain its therapeutic effects. The lessons learned from Carlumab’s development contribute to the broader field of monoclonal antibody research, influencing future strategies in targeting chemokines for disease treatment.
4.) Exploring Biosimilars for Carlumab
What is a Biosimilar?
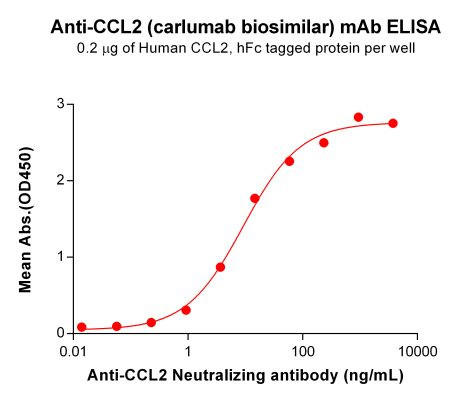
| Carlumab (Anti-CCL2) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | CCL2 |
| Reactivity: | Human |
How Does a Carlumab Biosimilar Compare to Carlumab?
Benefits of a Carlumab Biosimilar in Research
- Facilitates immunological and pharmacokinetic studies.
- Enables cost-effective preclinical testing.
- Supports the development of next-generation CCL2-targeting therapies.
Research Use Only Disclaimer:
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Chris McNally, PhD
Chris McNally, PhD, has a strong foundation in Biomedical Science, completing a PhD scholarship in collaboration with Randox Laboratories and Ulster University. Chris has published extensively in prostate cancer research, focusing on biomarker discovery, cancer risk stratification, and molecular mechanisms such as hypoxia-induced regulation. He currently serves as a Business Development Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



