Cabiralizumab: Unlocking the Potential of Anti-CSF1R Therapy
What You Need to Know About Cabiralizumab
What is Cabiralizumab?
Cabiralizumab is a monoclonal antibody targeting the colony-stimulating factor-1 receptor (CSF1R), developed for treating cancer and immune-mediated diseases.
What is the mechanism of action for Cabiralizumab?
Cabiralizumab works by inhibiting CSF1R signaling, reducing macrophage-mediated immune suppression in tumors and promoting anti-tumor immunity.
What are the clinical applications of Cabiralizumab?
It is being investigated for its potential in treating pancreatic cancer, tenosynovial giant cell tumor (TGCT), and immune-related diseases like PVNS.
1.) Understanding Cabiralizumab
Cabiralizumab (FPA008) is a monoclonal antibody designed to target and inhibit colony-stimulating factor 1 receptor (CSF1R), a critical regulator of macrophage survival, differentiation, and function. Macrophages play a vital role in maintaining immune homeostasis; however, in certain diseases, especially cancer, they can contribute to immune suppression and tumor progression. Tumor-associated macrophages (TAMs) are key players in this process, as they facilitate immune evasion and promote malignancy within the tumor microenvironment. By targeting CSF1R, cabiralizumab disrupts the activity of these macrophages, particularly within tumors, enhancing the immune response and limiting tumor progression.
Developed initially by Five Prime Therapeutics and later advanced through collaborations with Bristol-Myers Squibb, cabiralizumab has undergone extensive clinical trials, both as a monotherapy and in combination with other immunotherapies. Its primary application has been in oncology, especially in treating pancreatic cancer, an aggressive malignancy known for its highly immunosuppressive microenvironment. Preclinical and early clinical studies have shown that cabiralizumab, when used in combination with immune checkpoint inhibitors like nivolumab (Opdivo®), boosts anti-tumor immune responses, providing new therapeutic options for patients with limited treatments available.
In addition to its role in cancer therapy, cabiralizumab is also being explored for non-malignant conditions, such as pigmented villonodular synovitis (PVNS) and tenosynovial giant cell tumor (TGCT). In these diseases, abnormal macrophage accumulation drives pathological progression, and cabiralizumab’s ability to reduce macrophage numbers may alleviate symptoms and alter disease progression. As research into modulating the tumor microenvironment continues, cabiralizumab represents a significant advancement in both oncology and immune-mediated diseases, paving the way for further innovations in targeted therapies.
2.) Mechanism of Action of Cabiralizumab
Cabiralizumab exerts its therapeutic effects by selectively binding to colony-stimulating factor 1 receptor (CSF1R), a receptor expressed on macrophages and other myeloid cells crucial for their survival and function. CSF1R activation occurs through binding with its natural ligands, colony-stimulating factor 1 (CSF-1) and interleukin-34 (IL-34), which regulate macrophage differentiation, proliferation, and survival. By blocking CSF1R, cabiralizumab inhibits these signaling pathways, reducing macrophage recruitment and survival, especially in pathological contexts where macrophages contribute to disease progression.
In oncology, tumor-associated macrophages (TAMs) are a dominant component of the tumor microenvironment, where they play key roles in immune evasion. TAMs suppress cytotoxic T-cell activity, promote angiogenesis, and aid in tumor invasion and metastasis. Cabiralizumab disrupts these immunosuppressive functions by reducing TAM populations, thereby enhancing immune surveillance and fostering a more immune-permissive environment within the tumor. This is particularly significant in pancreatic cancer, where TAMs are integral to the disease’s immunosuppressive microenvironment, allowing tumors to escape immune detection.
When combined with immune checkpoint inhibitors like nivolumab, cabiralizumab enhances T-cell-mediated immune responses by relieving macrophage-induced immunosuppression. This dual mechanism—targeting macrophages while stimulating cytotoxic T-cell activity—offers a synergistic approach to cancer immunotherapy, improving treatment efficacy.
Beyond oncology, cabiralizumab’s inhibition of CSF1R is also relevant in diseases characterized by excessive macrophage activity, such as pigmented villonodular synovitis (PVNS) and tenosynovial giant cell tumor (TGCT). In these conditions, abnormal macrophage accumulation drives inflammation and tissue destruction. Cabiralizumab’s ability to modulate macrophage populations offers potential symptom relief and long-term disease control, addressing the root cause of these conditions.
3.) Clinical Applications of Cabiralizumab
Cabiralizumab has demonstrated promising potential across both oncology and non-oncology indications, particularly in diseases where macrophages contribute to disease progression.
Oncology Applications
In pancreatic cancer, one of the most aggressive and treatment-resistant malignancies, cabiralizumab is being investigated in combination with nivolumab. Pancreatic tumors create an immunosuppressive microenvironment dominated by tumor-associated macrophages, which inhibit anti-tumor immunity. Early-phase clinical trials suggest that cabiralizumab can reduce this macrophage population, potentially making tumors more responsive to immunotherapy. While initial results have shown promise, further research is needed to optimize treatment regimens and identify patient populations most likely to benefit.
Cabiralizumab is also being explored for other cancers, including glioblastoma and certain sarcomas, where macrophages play a significant role in tumor progression. Combination strategies with checkpoint inhibitors or chemotherapy are being studied to maximize therapeutic benefit.
Non-Oncology Applications
Beyond cancer, cabiralizumab has been evaluated in non-malignant diseases characterized by excessive macrophage activity. In pigmented villonodular synovitis (PVNS) and tenosynovial giant cell tumor (TGCT), abnormal macrophage accumulation leads to chronic inflammation and joint damage. Clinical trials have demonstrated that cabiralizumab can reduce lesion size and improve symptoms, making it a promising treatment option for these debilitating conditions.
Future Directions
While some clinical studies have encountered challenges, they have provided valuable insights into cabiralizumab’s mechanism and optimal therapeutic applications. Ongoing research aims to refine its role in combination therapies, explore additional cancer types, and investigate its potential in other immune-mediated diseases. With its unique approach to targeting macrophages, cabiralizumab remains a critical player in the evolving landscape of immunotherapy and precision medicine.
4.) Advancing Research on Cabiralizumab: The Role of Biosimilars
What is a Biosimilar?
A biosimilar is a highly similar version of an original biologic drug, developed to match its safety, efficacy, and quality. These products are invaluable in research, providing cost-effective alternatives for studying therapeutic mechanisms and applications.
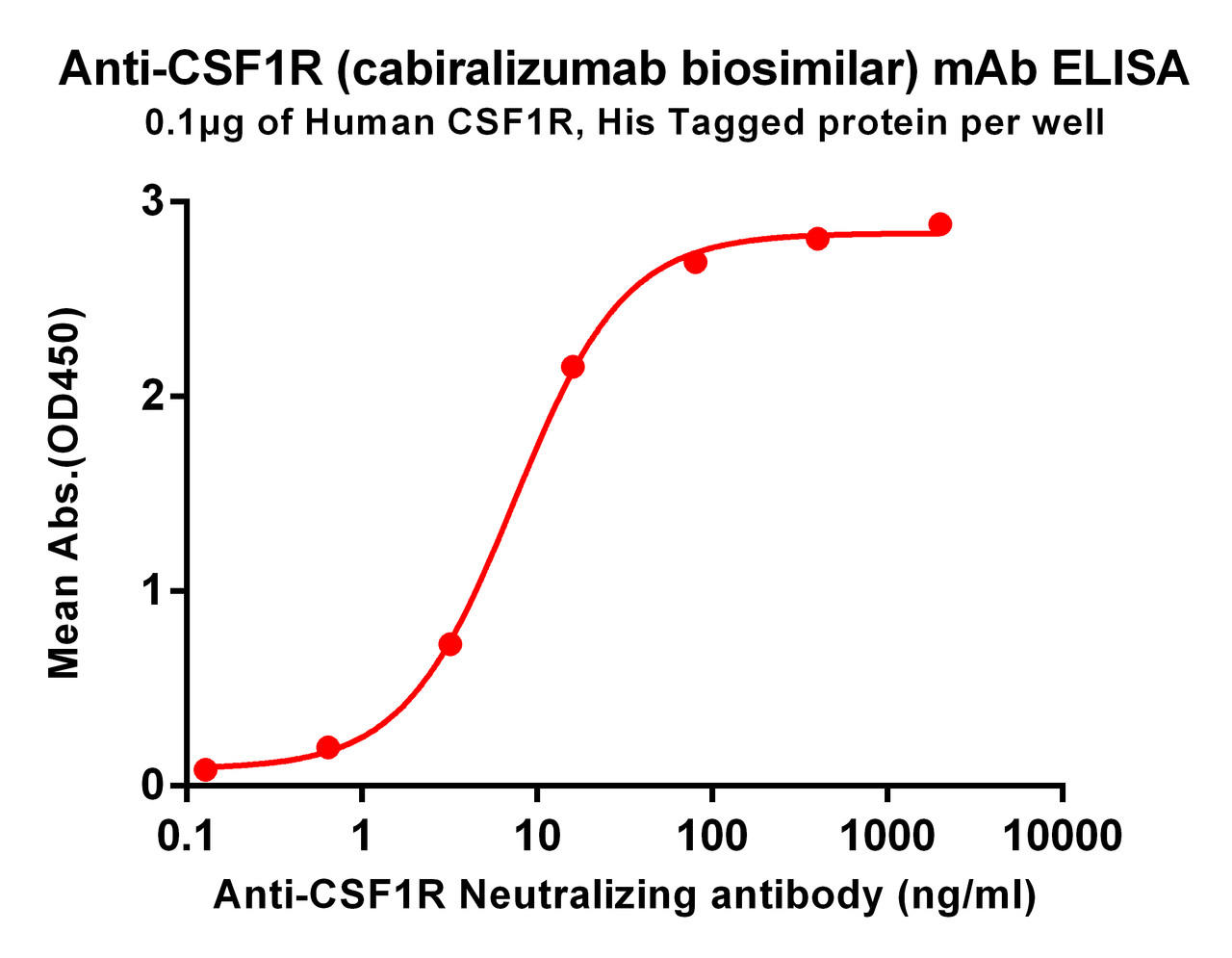
| Cabiralizumab (Anti-CSF1R) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | CSF1R |
| Reactivity: | Human |
Comparison: Cabiralizumab vs. Cabiralizumab Biosimilar
Cabiralizumab biosimilars replicate the original’s structure and function, ensuring comparable performance in preclinical and clinical settings. While the original is central to therapeutic applications, biosimilars offer a practical solution for research use, enabling broader investigation without the financial and regulatory constraints of proprietary drugs.
Benefits of Cabiralizumab Biosimilars
Cabiralizumab biosimilars serve as essential tools for studying CSF1R pathways, evaluating combination therapies, and exploring new applications. Their accessibility accelerates innovation, allowing researchers to conduct extensive studies that inform clinical strategies and drug development.
Research Use Only Disclaimer:
Cabiralizumab biosimilars are intended solely for research purposes and are not approved for clinical or therapeutic use.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Miren Ruiz de Eguilaz, PhD
Miren Ruiz de Eguilaz, PhD, has an extensive academic background, earning a BSc in Biology from UPV/EHU, an MSc in Biotechnology from the University of Oviedo, and a PhD in Chemistry from Dublin City University (DCU). Miren’s expertise lies in biosensor technology and bacterial diagnostics. She currently serves as a Product Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



