Brodalumab: A Breakthrough in IL-17 Receptor Blockade for Psoriasis and Beyond
Quick Facts About Brodalumab
What is Brodalumab?
Brodalumab is a monoclonal antibody that targets the IL-17 receptor to treat immune-mediated conditions like moderate-to-severe plaque psoriasis.
What is the mechanism of action of Brodalumab?
It blocks IL-17 receptor A, inhibiting downstream signaling pathways involved in inflammatory responses.
What conditions does Brodalumab treat?
Brodalumab is approved for psoriasis, psoriatic arthritis, and is being studied for other autoimmune disorders.
Is Brodalumab safe?
While generally well-tolerated, it carries a REMS program due to risks of suicidal ideation in some patients. Always consult healthcare professionals for guidance.
1.) Understanding Brodalumab
Brodalumab is a groundbreaking biologic therapy that has redefined the treatment of immune-mediated diseases, particularly moderate-to-severe plaque psoriasis. Initially developed by Amgen, its commercial journey involved partnerships with pharmaceutical giants such as AstraZeneca and Valeant Pharmaceuticals, now Bausch Health. This collaboration underscores the significant potential of Brodalumab in transforming the management of inflammatory conditions driven by immune system overactivation.
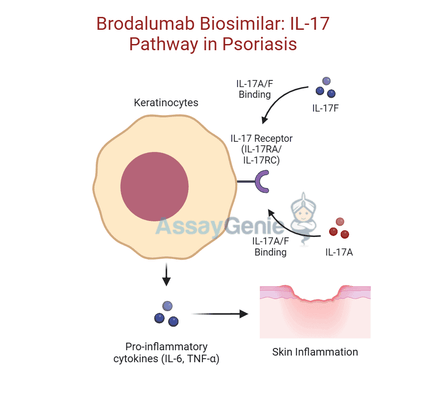
What sets Brodalumab apart from other biologics is its unique mechanism of action. Unlike therapies that inhibit individual cytokines such as IL-17A or IL-17F, Brodalumab specifically targets the IL-17 receptor A (IL-17RA). By blocking this receptor, Brodalumab effectively prevents the action of multiple IL-17 cytokines, which play a central role in inflammatory pathways. This receptor-focused approach results in a broader suppression of the inflammatory cascade, leading to substantial reductions in plaque formation, skin inflammation, and other symptoms in psoriasis patients.
The efficacy of Brodalumab has been demonstrated through clinical trials, with many patients achieving rapid and complete symptom relief, including PASI 100 scores. Its benefits extend beyond psoriasis, as emerging research investigates its potential for treating other immune-mediated diseases, such as hidradenitis suppurativa, systemic sclerosis, and asthma. These ongoing studies suggest Brodalumab’s utility may span a wide range of conditions characterized by dysregulated IL-17 signaling.
With its innovative mechanism of action, expanding indications, and proven efficacy, Brodalumab represents the forefront of biologic therapy, offering new hope to patients with chronic inflammatory diseases and exemplifying the advancements in immunology-driven treatment strategies.
2.) Mechanism of Action of Brodalumab
Brodalumab is a human monoclonal antibody designed to target interleukin-17 receptor A (IL-17RA), a crucial component of the IL-17 cytokine family’s signaling cascade. This receptor mediates the effects of several pro-inflammatory cytokines, including IL-17A, IL-17F, and IL-17E. By selectively binding to IL-17RA, Brodalumab blocks the downstream signaling pathways that drive inflammation, keratinocyte proliferation, and immune cell recruitment. This mechanism disrupts the pathological processes that underlie autoimmune and inflammatory conditions such as psoriasis.
Unlike therapies that target individual cytokines like IL-17A or IL-17F, Brodalumab’s receptor-focused approach provides a broader spectrum of inhibition across the IL-17 family. This comprehensive blockade ensures a more robust and sustained suppression of inflammation, making it particularly effective for severe cases of plaque psoriasis and other IL-17-driven diseases.
- Rapid Symptom Relief: Clinical trials show that Brodalumab provides rapid clearance of psoriatic plaques, with a significant proportion of patients achieving PASI 75, PASI 90, or even complete skin clearance (PASI 100) within weeks of initiation.
- Comprehensive Targeting: The IL-17RA blockade interrupts multiple inflammatory pathways, distinguishing Brodalumab from narrower therapeutic approaches.
- Emerging Applications: Beyond psoriasis, Brodalumab is being explored for conditions such as psoriatic arthritis, asthma, hidradenitis suppurativa, and systemic sclerosis, demonstrating its potential across a range of IL-17-mediated diseases.
By targeting the receptor itself, Brodalumab offers a unique and effective strategy in the therapeutic arsenal for inflammatory and autoimmune conditions, setting it apart from traditional cytokine inhibitors.

3.) Clinical Applications of Brodalumab
1. Psoriasis
Brodalumab is FDA-approved for the treatment of moderate-to-severe plaque psoriasis in adults. Clinical trials, including the pivotal AMAGINE series, highlighted its efficacy, with high proportions of patients achieving complete skin clearance.
2. Psoriatic Arthritis
While primarily approved for psoriasis, Brodalumab has shown promise in reducing joint inflammation and improving physical function in patients with psoriatic arthritis.
3. Emerging Areas
- Hidradenitis Suppurativa: Early investigations suggest benefits in reducing lesion formation and inflammatory activity.
- Asthma: By modulating IL-17-driven inflammation, Brodalumab may offer therapeutic value in severe, treatment-resistant asthma.
- Systemic Sclerosis: As a fibrotic condition linked to IL-17 activity, systemic sclerosis represents another potential target for Brodalumab therapy.
4. Safety Considerations
Brodalumab is generally well-tolerated but carries a REMS requirement due to reports of suicidal ideation. Its safety profile underscores the importance of careful patient monitoring.
4.) Exploring Biosimilars for Brodalumab
What is a Biosimilar?
A biosimilar is a biologic product that closely matches an existing FDA-approved biologic in terms of safety, efficacy, and quality. Biosimilars play a vital role in expanding research opportunities by providing cost-effective alternatives for study and discovery.
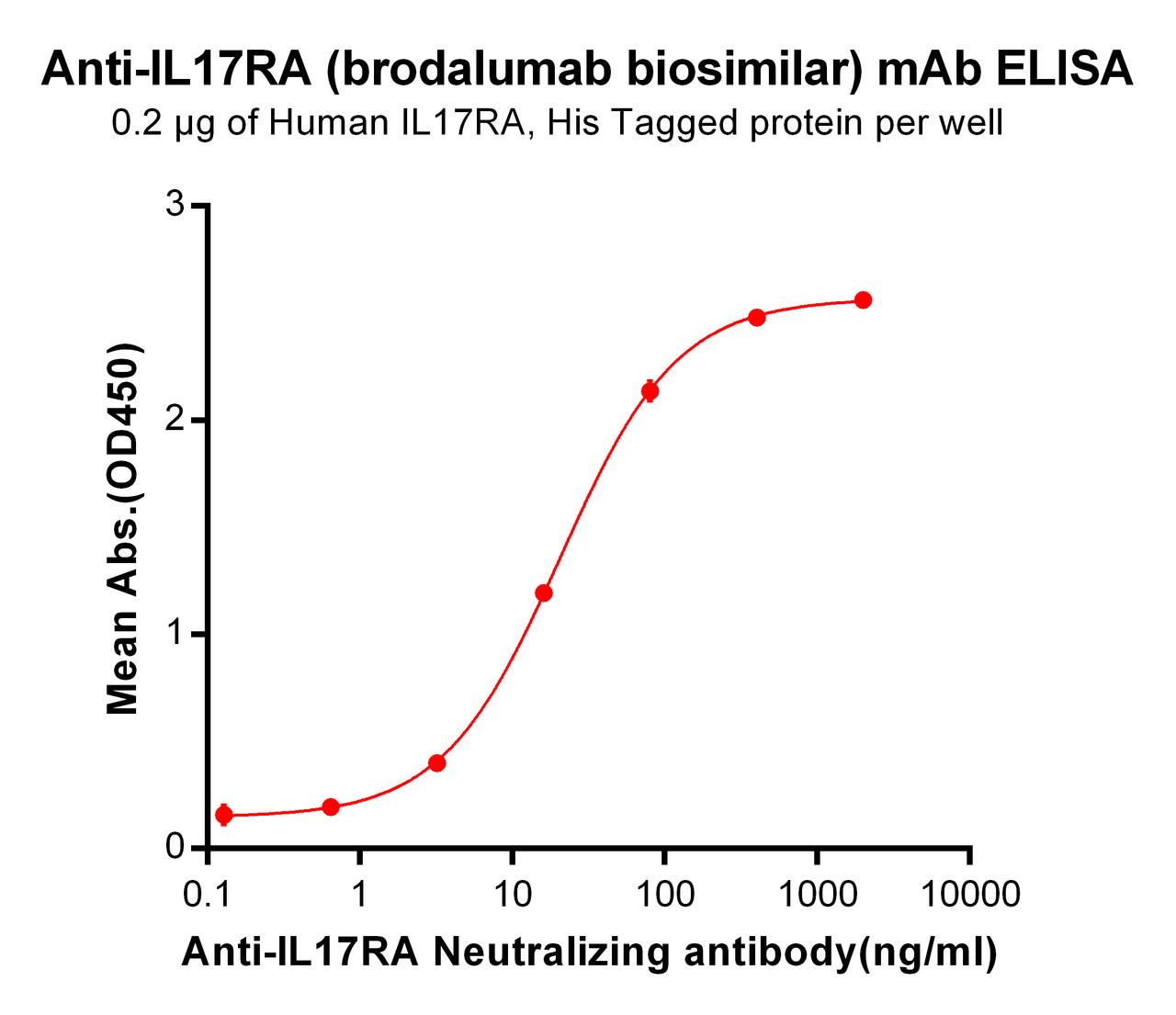
| Brodaluamab (Anti-IL17RA) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | IL-17RA |
| Reactivity: | Human |
How Brodalumab Biosimilar Compares
Brodalumab biosimilars replicate the original product's IL-17 receptor-blocking mechanism, offering comparable efficacy in research contexts. These biosimilars provide researchers with valuable tools for studying immunological pathways and testing innovative therapeutic approaches.
Key Benefits of Brodalumab Biosimilars
2. Advancing Research: Facilitates preclinical and translational studies to explore novel applications.
3. Consistency: Offers a reliable platform for studying IL-17 signaling in inflammatory diseases.
Research Use Only Disclaimer:
Brodalumab biosimilars are intended for research purposes only and are not approved for clinical use in patients.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Miren Ruiz de Eguilaz, PhD
Miren Ruiz de Eguilaz, PhD, has an extensive academic background, earning a BSc in Biology from UPV/EHU, an MSc in Biotechnology from the University of Oviedo, and a PhD in Chemistry from Dublin City University (DCU). Miren’s expertise lies in biosensor technology and bacterial diagnostics. She currently serves as a Product Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025