Bococizumab: Mechanism, Clinical Applications, and Biosimilars in Research
Quick Facts About Bococizumab
What is Bococizumab?
How Does Bococizumab Work?
Was Bococizumab Approved for Use?
What Are the Side Effects of Bococizumab?
Clinical trials reported increased anti-drug antibodies, leading to reduced efficacy over time.
1.) Understanding Bococizumab
Bococizumab was developed as a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, a class of drugs designed to lower low-density lipoprotein (LDL) cholesterol levels. It was intended for patients with hypercholesterolemia and high cardiovascular risk who required additional cholesterol reduction beyond standard lipid-lowering therapies such as statins. Bococizumab was developed by Pfizer and entered clinical trials as a promising therapeutic alternative to Alirocumab and Evolocumab, two other monoclonal antibodies targeting PCSK9.
Unlike its competitors, Bococizumab was a partially humanized monoclonal antibody, meaning it contained both human and non-human elements in its structure. While this design aimed to improve its functionality, it inadvertently contributed to the drug's immunogenicity, which became a significant challenge during clinical trials. The development of anti-drug antibodies (ADAs) in many patients led to reduced drug efficacy over time, making it less reliable compared to fully humanized PCSK9 inhibitors.
Despite initial optimism, Pfizer discontinued Bococizumab’s development after results from the SPIRE clinical trials showed considerable variability in lipid-lowering effects. This inconsistency was especially pronounced in patients who developed ADAs, leading to diminished long-term efficacy. The high incidence of immunogenic reactions raised concerns about its viability as a long-term therapeutic option. Nevertheless, Bococizumab’s development has contributed to the broader understanding of PCSK9 inhibition in cholesterol management, and its challenges have influenced the design of newer, more effective lipid-lowering therapies. The case of Bococizumab highlights the importance of immunogenicity assessments in monoclonal antibody development and underscores the role of PCSK9 inhibitors in cardiovascular disease management.
2.) Mechanism of Action of Bococizumab
Bococizumab functions by binding to proprotein convertase subtilisin/kexin type 9 (PCSK9), a protein involved in the regulation of LDL receptors. PCSK9 normally binds to LDL receptors on the surface of liver cells, targeting them for degradation. This process reduces the number of LDL receptors available to clear LDL cholesterol from the bloodstream, leading to elevated cholesterol levels. By inhibiting PCSK9, Bococizumab prevents this degradation, allowing more LDL receptors to remain functional, thereby increasing LDL clearance and lowering cholesterol levels.
While this mechanism is consistent with other PCSK9 inhibitors like Alirocumab and Evolocumab, Bococizumab’s partial humanization contributed to a high incidence of anti-drug antibody (ADA) formation. ADAs are immune system responses against therapeutic proteins that can neutralize drug activity or accelerate drug clearance. In clinical trials, many patients developed ADAs, leading to a gradual decline in Bococizumab’s cholesterol-lowering effects. This immunogenic response was a key factor in its discontinuation, as it resulted in greater variability in efficacy compared to other PCSK9 inhibitors.
Despite its discontinuation, Bococizumab's research has provided valuable insights into PCSK9 inhibition and cholesterol metabolism. It demonstrated the potential benefits of targeting PCSK9 but also highlighted the risks of immunogenicity in monoclonal antibody therapies. The findings from Bococizumab’s trials have influenced the development of newer lipid-lowering agents, emphasizing the need for fully humanized antibodies to minimize ADA-related efficacy loss. Furthermore, ongoing research into small-molecule PCSK9 inhibitors and alternative lipid-lowering strategies continues to build on the foundation laid by Bococizumab and its predecessors.
3.) Clinical Applications of Bococizumab
Bococizumab was initially developed for patients with hypercholesterolemia and those at high risk of cardiovascular disease who required additional LDL cholesterol reduction beyond standard treatments. It was investigated in clinical trials as an adjunct therapy for patients who did not achieve optimal cholesterol control with statins alone. The SPIRE (Studies of PCSK9 Inhibition and the Reduction of Vascular Events) clinical trial program was launched to evaluate Bococizumab’s efficacy and safety across different patient populations.
The SPIRE-1 and SPIRE-2 trials assessed the drug’s ability to reduce cardiovascular events in high-risk patients. While Bococizumab initially demonstrated promising LDL cholesterol-lowering effects, the emergence of ADAs led to a significant reduction in efficacy over time. Patients who developed ADAs experienced a diminished response, resulting in inconsistent lipid reduction across the study population. This variability in treatment outcomes was a major concern and contributed to Pfizer’s decision to halt Bococizumab’s development.
Another issue identified during the trials was the potential for increased adverse effects compared to fully humanized PCSK9 inhibitors. The high immunogenicity of Bococizumab not only reduced its effectiveness but also raised concerns about its long-term safety profile. Given these findings, Pfizer discontinued the program, shifting focus to alternative cardiovascular therapies.
Despite its discontinuation, Bococizumab’s clinical research has been instrumental in shaping the future of PCSK9-targeting therapies. It underscored the importance of immunogenicity assessments in monoclonal antibody development and highlighted the need for fully humanized antibodies to ensure sustained efficacy. The lessons learned from Bococizumab continue to influence the design of next-generation lipid-lowering therapies, ensuring better patient outcomes in cardiovascular disease management.
4.) Exploring Biosimilars for Bococizumab
What is a Biosimilar?
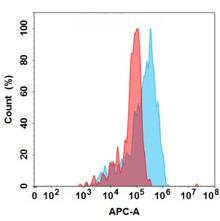
| Bococizumab (Anti-PCSK9) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | PCSK9 |
| Reactivity: | Human |
Bococizumab Biosimilar: Advancing Research
Although Bococizumab was discontinued, biosimilars serve as valuable tools for studying PCSK9 inhibition, providing researchers with access to alternatives for non-clinical investigations.
Comparison: Bococizumab vs. Its Biosimilar
Research Use Only Disclaimer:
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Marina Alberto, PhD
Marina Alberto, PhD, holds a robust academic background in Biotechnology, earning her Bachelor’s Degree and PhD in Science and Technology from Quilmes National University. Her research spans cancer immunotherapy, glycan profiling, and vaccine development, including innovative projects on pediatric leukemia diagnosis and cancer-associated carbohydrate-mimetic vaccines. She currently serves as a Technical Support and Sales Specialist at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



