Bermekimab: Unveiling the Role of Anti-CD47 in Hidradenitis Suppurativa and Atopic Dermatitis
What You Need to Know About Bermekimab
What is Bermekimab?
Bermekimab is a monoclonal antibody targeting the CD47 receptor, developed to modulate the immune system and improve conditions like hidradenitis suppurativa and atopic dermatitis.
What is the mechanism of action for Bermekimab?
Bermekimab blocks the CD47 pathway, helping to enhance immune responses, which is particularly beneficial in treating inflammatory skin conditions and potentially cancer.
What are the clinical applications of Bermekimab?
Bermekimab is being investigated for use in hidradenitis suppurativa and atopic dermatitis, with promising clinical trial outcomes suggesting it may address unmet needs in these areas.
1.) Understanding Bermekimab
Bermekimab is a novel monoclonal antibody designed to target CD47, a receptor that plays a critical role in regulating the immune system. CD47 is known as a “don’t eat me” signal to macrophages and other immune cells, helping to protect normal cells from being targeted and destroyed. This immune modulation pathway has primarily been studied in the context of cancer immunotherapy, where tumors exploit CD47 to evade immune surveillance. Bermekimab disrupts this signaling pathway by binding to CD47, effectively blocking its “don’t eat me” signal, thereby enabling the immune system to recognize and eliminate disease-causing cells more efficiently.
While Bermekimab’s mechanism of action is rooted in immune checkpoint regulation—a strategy often employed in cancer therapies—its application extends beyond oncology. This innovative approach positions Bermekimab as a promising candidate for treating chronic inflammatory diseases like hidradenitis suppurativa (HS) and atopic dermatitis (AD), conditions for which existing treatments may be inadequate. In HS, which involves painful skin lesions and recurrent flare-ups, and in AD, characterized by chronic itching and dry skin, Bermekimab’s potential to modulate the immune system could offer significant therapeutic benefits, especially for patients with severe, refractory symptoms.
Emerging research, including phase 2 and 3 clinical trials, has demonstrated Bermekimab’s effectiveness in treating these challenging skin conditions, particularly in patients who do not respond well to conventional therapies. As the clinical development of Bermekimab progresses, its broader therapeutic applications continue to expand, offering new hope for patients with persistent, complex inflammatory conditions that are difficult to manage with current therapies.
2.) Mechanism of Action of Bermekimab
Bermekimab’s therapeutic action hinges on its ability to selectively target CD47, a cell-surface glycoprotein that is expressed on various cell types, including immune cells and tumor cells. CD47 is often referred to as the “don’t eat me” signal because it binds to a receptor on macrophages, preventing them from engulfing and destroying cells that express CD47. This mechanism is essential for protecting normal cells from immune attack, but tumors have also been shown to exploit CD47 expression to avoid immune detection.
By binding to CD47, Bermekimab effectively blocks this protective signal, preventing it from interacting with macrophages and other immune cells. This inhibition promotes enhanced phagocytosis, where immune cells, particularly macrophages, are able to identify and clear disease-causing cells more efficiently. Bermekimab’s ability to reverse immune suppression has profound implications for various diseases, including autoimmune conditions, chronic inflammatory skin diseases, and potentially even cancer.
For patients with conditions like hidradenitis suppurativa and atopic dermatitis, where the immune system becomes dysregulated and leads to chronic inflammation, Bermekimab offers a new avenue of treatment. Studies suggest that by modulating immune responses, Bermekimab can reduce inflammation and alleviate symptoms, such as painful lesions in HS and persistent skin irritation in AD. The drug’s potential to enhance immune system activation while targeting the root cause of inflammation could make it a valuable treatment for patients whose conditions are resistant to current therapies.
3.) Clinical Applications of Bermekimab
Bermekimab is currently undergoing clinical trials for a range of inflammatory skin disorders, including hidradenitis suppurativa (HS) and atopic dermatitis (AD). These conditions are characterized by chronic, often debilitating inflammation that can severely impact patients’ quality of life. In HS, patients experience painful abscesses and tunnels under the skin, leading to frequent flare-ups and scarring. Traditional treatments, such as antibiotics, immunosuppressants, and biologics, may not always provide adequate relief, especially in severe cases. Bermekimab’s mechanism of action offers a novel therapeutic approach by enhancing immune system activity, potentially reducing the frequency and severity of flare-ups in HS patients.
In atopic dermatitis, a common skin condition characterized by dry, itchy patches of skin, patients often struggle with persistent inflammation despite treatment with topical steroids and other immunosuppressive therapies. Bermekimab’s immune-modulating properties may help to alleviate the chronic inflammation seen in AD, reducing symptoms such as itching and skin irritation. Clinical trials have shown promising results for Bermekimab in AD patients who are unresponsive to conventional therapies, suggesting that the drug could provide significant symptom relief and improve patients’ quality of life.
As Bermekimab continues to undergo rigorous clinical testing, its potential extends beyond skin conditions. Researchers are also exploring its role in treating other autoimmune diseases, where immune dysregulation plays a central role in disease pathogenesis. Bermekimab could offer a groundbreaking alternative for individuals with persistent, difficult-to-treat autoimmune conditions, providing a new treatment option that directly targets the immune system’s overactive response.
The ongoing development of Bermekimab signals an exciting shift in the treatment landscape for inflammatory diseases, offering hope for patients suffering from chronic conditions that have previously been difficult to manage. With further research and clinical trials, Bermekimab could soon become a key player in the therapeutic arsenal for chronic inflammatory diseases and autoimmune disorders.
4.) How Bermekimab Biosimilar Compares to Bermekimab
What is a Biosimilar?
A biosimilar is a biologic medical product highly similar to an already-approved reference product, with no clinically meaningful differences in terms of safety, purity, and potency. Biosimilars provide an affordable alternative to their reference drugs, which is especially beneficial in enhancing research accessibility and providing more options for drug development.
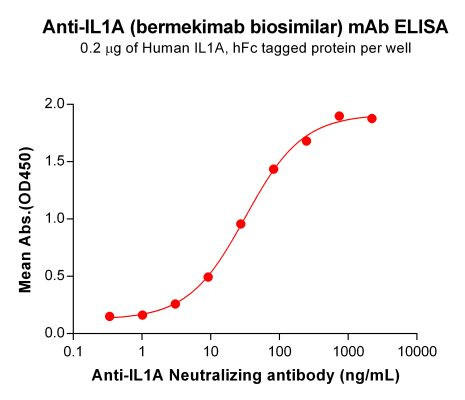
| Bermekimab (Anti-IL1A) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | IL-1A |
| Reactivity: | Human |
Advancing Research on Bermekimab
The introduction of Bermekimab’s biosimilar offers an important tool for ongoing scientific studies. Research applications for the biosimilar mirror the therapeutic potential of Bermekimab, enabling scientists to conduct cost-effective investigations into CD47 inhibition and its role in autoimmune diseases, chronic inflammation, and cancer therapies. By providing a reliable alternative for research, the Bermekimab biosimilar supports the continued exploration of its mechanisms in both preclinical and clinical settings.
Comparison to Bermekimab
Benefits of Bermekimab Biosimilar in Research
The Bermekimab biosimilar has particular value in accelerating research, providing an essential tool for investigating chronic inflammatory conditions like hidradenitis suppurativa and atopic dermatitis. By utilizing a more accessible alternative to Bermekimab, researchers can better explore therapeutic strategies for these conditions, contributing to the development of more effective treatments for patients worldwide.
Research Use Only Disclaimer:
The Bermekimab biosimilar is intended solely for research purposes and should not be used for therapeutic purposes in clinical settings.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Marina Alberto, PhD
Marina Alberto, PhD, holds a robust academic background in Biotechnology, earning her Bachelor’s Degree and PhD in Science and Technology from Quilmes National University. Her research spans cancer immunotherapy, glycan profiling, and vaccine development, including innovative projects on pediatric leukemia diagnosis and cancer-associated carbohydrate-mimetic vaccines. She currently serves as a Technical Support and Sales Specialist at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



